200,000+ products from a single source!
sales@angenechem.com
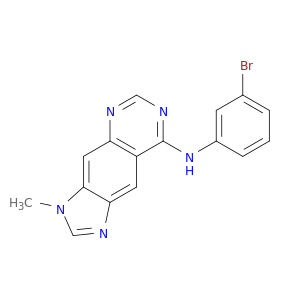
174709-30-9 | 3H-Imidazo[4,5-g]quinazolin-8-amine, N-(3-bromophenyl)-3-methyl-
CAS No: 174709-30-9 Catalog No: AG001ZVW MDL No:
Product Description
Catalog Number:
AG001ZVW
Chemical Name:
3H-Imidazo[4,5-g]quinazolin-8-amine, N-(3-bromophenyl)-3-methyl-
CAS Number:
174709-30-9
Molecular Formula:
C16H12BrN5
Molecular Weight:
354.2040
IUPAC Name:
N-(3-bromophenyl)-3-methylimidazo[4,5-g]quinazolin-8-amine
InChI:
InChI=1S/C16H12BrN5/c1-22-9-20-14-6-12-13(7-15(14)22)18-8-19-16(12)21-11-4-2-3-10(17)5-11/h2-9H,1H3,(H,18,19,21)
InChI Key:
YAMAGACQNDAKFB-UHFFFAOYSA-N
SMILES:
Brc1cccc(c1)Nc1ncnc2c1cc1ncn(c1c2)C
Properties
Complexity:
395
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
353.028g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
354.211g/mol
Monoisotopic Mass:
353.028g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
55.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.6
Literature
| Title | Journal |
|---|---|
| A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. | The Biochemical journal 20130415 |
| Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. | Nature biotechnology 20111101 |
| Role of a tyrosine kinase in the CO2-induced stimulation of HCO3- reabsorption by rabbit S2 proximal tubules. | American journal of physiology. Renal physiology 20060801 |
| Tyrosine kinase inhibitors. 9. Synthesis and evaluation of fused tricyclic quinazoline analogues as ATP site inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. | Journal of medicinal chemistry 19960216 |
Related Products
© 2019 Angene International Limited. All rights Reserved.