200,000+ products from a single source!
sales@angenechem.com
Home > Boronic Acids > 174671-46-6
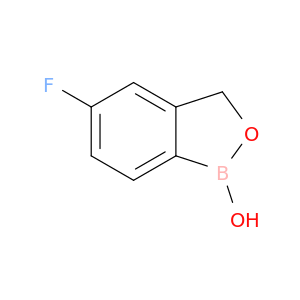
174671-46-6 | 2,1-Benzoxaborole, 5-fluoro-1,3-dihydro-1-hydroxy-
CAS No: 174671-46-6 Catalog No: AG001ZVO MDL No:MFCD10699483
Product Description
Catalog Number:
AG001ZVO
Chemical Name:
2,1-Benzoxaborole, 5-fluoro-1,3-dihydro-1-hydroxy-
CAS Number:
174671-46-6
Molecular Formula:
C7H6BFO2
Molecular Weight:
151.9307
MDL Number:
MFCD10699483
IUPAC Name:
5-fluoro-1-hydroxy-3H-2,1-benzoxaborole
InChI:
InChI=1S/C7H6BFO2/c9-6-1-2-7-5(3-6)4-11-8(7)10/h1-3,10H,4H2
InChI Key:
LFQDNHWZDQTITF-UHFFFAOYSA-N
SMILES:
Fc1ccc2c(c1)COB2O
UNII:
K124A4EUQ3
Properties
Complexity:
155
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
152.044g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
151.931g/mol
Monoisotopic Mass:
152.044g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
29.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Tavaborole: first global approval. | Drugs 20140901 |
| The efficacy and safety of tavaborole, a novel, boron-based pharmaceutical agent: phase 2 studies conducted for the topical treatment of toenail onychomycosis. | Journal of drugs in dermatology : JDD 20140901 |
| Characterization of benzoxaborole-based antifungal resistance mutations demonstrates that editing depends on electrostatic stabilization of the leucyl-tRNA synthetase editing cap. | FEBS letters 20111003 |
| Boron-containing inhibitors of synthetases. | Chemical Society reviews 20110801 |
| 5-Fluoro-1,3-dihydro-2,1-benzoxaborol-1-ol. | Acta crystallographica. Section E, Structure reports online 20110201 |
| Post-transfer editing by a eukaryotic leucyl-tRNA synthetase resistant to the broad-spectrum drug AN2690. | The Biochemical journal 20100901 |
| Crystal structures of the human and fungal cytosolic Leucyl-tRNA synthetase editing domains: A structural basis for the rational design of antifungal benzoxaboroles. | Journal of molecular biology 20090710 |
| A genomic glimpse of aminoacyl-tRNA synthetases in malaria parasite Plasmodium falciparum. | BMC genomics 20090101 |
| In Vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. | Journal of pharmaceutical sciences 20071001 |
| AN-2690, a novel antifungal for the topical treatment of onychomycosis. | Current opinion in investigational drugs (London, England : 2000) 20070801 |
| An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. | Science (New York, N.Y.) 20070622 |
| Recent progress on the topical therapy of onychomycosis. | Expert opinion on investigational drugs 20070201 |
| Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the potential treatment of onychomycosis. | Journal of medicinal chemistry 20060727 |
| Spotlight on targeting aminoacyl-tRNA synthetases for the treatment of fungal infections. | Drug news & perspectives 20060101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.