200,000+ products from a single source!
sales@angenechem.com
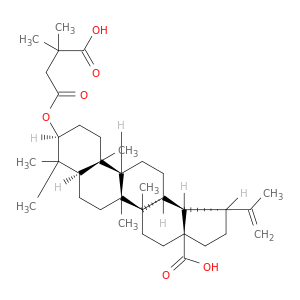
174022-42-5 | Lup-20(29)-en-28-oic acid, 3-(3-carboxy-3-methyl-1-oxobutoxy)-, (3β)-
CAS No: 174022-42-5 Catalog No: AG001ZEC MDL No:MFCD00954243
Product Description
Catalog Number:
AG001ZEC
Chemical Name:
Lup-20(29)-en-28-oic acid, 3-(3-carboxy-3-methyl-1-oxobutoxy)-, (3β)-
CAS Number:
174022-42-5
Molecular Formula:
C36H56O6
Molecular Weight:
584.8262
MDL Number:
MFCD00954243
IUPAC Name:
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-carboxy-3-methylbutanoyl)oxy-5a,5b,8,8,11a-pentamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carboxylic acid
InChI:
InChI=1S/C36H56O6/c1-21(2)22-12-17-36(30(40)41)19-18-34(8)23(28(22)36)10-11-25-33(7)15-14-26(42-27(37)20-31(3,4)29(38)39)32(5,6)24(33)13-16-35(25,34)9/h22-26,28H,1,10-20H2,2-9H3,(H,38,39)(H,40,41)/t22-,23+,24-,25+,26-,28+,33-,34+,35+,36-/m0/s1
InChI Key:
YJEJKUQEXFSVCJ-WRFMNRASSA-N
SMILES:
O=C(CC(C(=O)O)(C)C)O[C@H]1CC[C@]2([C@H](C1(C)C)CC[C@@]1([C@@H]2CC[C@H]2[C@@]1(C)CC[C@@]1([C@@H]2[C@@H](CC1)C(=C)C)C(=O)O)C)C
EC Number:
605-702-6
UNII:
S125DW66N8
Properties
Complexity:
1170
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
10
Defined Bond Stereocenter Count:
0
Exact Mass:
584.408g/mol
Formal Charge:
0
Heavy Atom Count:
42
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
584.838g/mol
Monoisotopic Mass:
584.408g/mol
Rotatable Bond Count:
7
Topological Polar Surface Area:
101A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
9.1
Literature
| Title | Journal |
|---|---|
| Incorporation of Privileged Structures into Bevirimat Can Improve Activity against Wild-Type and Bevirimat-Resistant HIV-1. | Journal of medicinal chemistry 20161013 |
| Identification and Characterization of BMS-955176, a Second-Generation HIV-1 Maturation Inhibitor with Improved Potency, Antiviral Spectrum, and Gag Polymorphic Coverage. | Antimicrobial agents and chemotherapy 20160701 |
| Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity. | ACS medicinal chemistry letters 20160609 |
| C-3 benzoic acid derivatives of C-3 deoxybetulinic acid and deoxybetulin as HIV-1 maturation inhibitors. | Bioorganic & medicinal chemistry 20160415 |
| Inhibitors of HIV-1 maturation: Development of structure-activity relationship for C-28 amides based on C-3 benzoic acid-modified triterpenoids. | Bioorganic & medicinal chemistry letters 20160415 |
| Fluorinated betulinic acid derivatives and evaluation of their anti-HIV activity. | Bioorganic & medicinal chemistry letters 20160101 |
| Isolation, Structural Modification, and HIV Inhibition of Pentacyclic Lupane-Type Triterpenoids from Cassine xylocarpa and Maytenus cuzcoina. | Journal of natural products 20150522 |
| Synthesis and biological evaluation of a new derivative of bevirimat that targets the Gag CA-SP1 cleavage site. | European journal of medicinal chemistry 20130401 |
| New betulinic acid derivatives for bevirimat-resistant human immunodeficiency virus type-1. | Journal of medicinal chemistry 20130314 |
| Anti-AIDS agents 90. novel C-28 modified bevirimat analogues as potent HIV maturation inhibitors. | Journal of medicinal chemistry 20120927 |
| Synthesis of betulinic acid derivatives as entry inhibitors against HIV-1 and bevirimat-resistant HIV-1 variants. | Bioorganic & medicinal chemistry letters 20120815 |
| New betulinic acid derivatives as potent proteasome inhibitors. | Bioorganic & medicinal chemistry letters 20111001 |
| A single polymorphism in HIV-1 subtype C SP1 is sufficient to confer natural resistance to the maturation inhibitor bevirimat. | Antimicrobial agents and chemotherapy 20110701 |
| Efficient synthesis and biological evaluation of epiceanothic acid and related compounds. | Bioorganic & medicinal chemistry letters 20110101 |
| HIV-1 protease inhibitor mutations affect the development of HIV-1 resistance to the maturation inhibitor bevirimat. | Retrovirology 20110101 |
| The prototype HIV-1 maturation inhibitor, bevirimat, binds to the CA-SP1 cleavage site in immature Gag particles. | Retrovirology 20110101 |
| A fourth scale sensitive to the magnetic field; intermolecular frequency symmetry in a specific interaction between protein and low-molecular compound. | Chemical & pharmaceutical bulletin 20101201 |
| Lersivirine, a nonnucleoside reverse transcriptase inhibitor with activity against drug-resistant human immunodeficiency virus type 1. | Antimicrobial agents and chemotherapy 20101001 |
| Conjugates of betulin derivatives with AZT as potent anti-HIV agents. | Bioorganic & medicinal chemistry 20100901 |
| The capsid-spacer peptide 1 Gag processing intermediate is a dominant-negative inhibitor of HIV-1 maturation. | Virology 20100425 |
| Anti-AIDS agents 81. Design, synthesis, and structure-activity relationship study of betulinic acid and moronic acid derivatives as potent HIV maturation inhibitors. | Journal of medicinal chemistry 20100422 |
| Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach. | Journal of natural products 20100326 |
| High prevalence of bevirimat resistance mutations in protease inhibitor-resistant HIV isolates. | AIDS (London, England) 20100313 |
| Can the further clinical development of bevirimat be justified? | AIDS (London, England) 20100313 |
| [The role of structural protein Gag and related gene (protein) in late stages of the HIV-1 replication cycle and the inhibitors]. | Yao xue xue bao = Acta pharmaceutica Sinica 20100201 |
| High prevalence of natural polymorphisms in Gag (CA-SP1) associated with reduced response to Bevirimat, an HIV-1 maturation inhibitor. | AIDS (London, England) 20100128 |
| Predicting Bevirimat resistance of HIV-1 from genotype. | BMC bioinformatics 20100101 |
| Polymorphisms in Gag spacer peptide 1 confer varying levels of resistance to the HIV- 1 maturation inhibitor bevirimat. | Retrovirology 20100101 |
| [Prediction of the efficacy of bevirimat used for the treatment of HIV infection in Russia]. | Voprosy virusologii 20100101 |
| Betulinic acid derivatives as human immunodeficiency virus type 2 (HIV-2) inhibitors. | Journal of medicinal chemistry 20091210 |
| New small-molecule inhibitor class targeting human immunodeficiency virus type 1 virion maturation. | Antimicrobial agents and chemotherapy 20091201 |
| Cancer preventive agents 9. Betulinic acid derivatives as potent cancer chemopreventive agents. | Bioorganic & medicinal chemistry letters 20090701 |
| Anti-AIDS agents. 78. Design, synthesis, metabolic stability assessment, and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus (HIV) agents. | Journal of medicinal chemistry 20090528 |
| An oral human drug absorption study to assess the impact of site of delivery on the bioavailability of bevirimat. | Journal of clinical pharmacology 20090501 |
| [New antiretroviral drug classes in HIV therapy]. | MMW Fortschritte der Medizin 20090430 |
| Drug interactions with new and investigational antiretrovirals. | Clinical pharmacokinetics 20090101 |
| Study may point way forward for bevirimat. | Project Inform perspective 20081201 |
| Report from the 2008 joint ICAAC/IDSA meeting. Bevirimat: predictors of response. | AIDS clinical care 20081201 |
| The absorption, distribution, metabolism and elimination of bevirimat in rats. | Biopharmaceutics & drug disposition 20081001 |
| Pharmacokinetic properties and tolerability of bevirimat and atazanavir in healthy volunteers: an open-label, parallel-group study. | Clinical therapeutics 20081001 |
| New drugs. | Journal of HIV therapy 20080601 |
| Bevirimat: a novel maturation inhibitor for the treatment of HIV-1 infection. | Antiviral chemistry & chemotherapy 20080101 |
| The inhibition of assembly of HIV-1 virus-like particles by 3-O-(3',3'-dimethylsuccinyl) betulinic acid (DSB) is counteracted by Vif and requires its Zinc-binding domain. | Virology journal 20080101 |
| The maturation inhibitor bevirimat (PA-457) can be active in patients carrying HIV type-1 non-B subtypes and recombinants. | Antiviral therapy 20080101 |
| Anti-AIDS agents 73: structure-activity relationship study and asymmetric synthesis of 3-O-monomethylsuccinyl-betulinic acid derivatives. | Bioorganic & medicinal chemistry letters 20071201 |
| Activation and inhibition of the proteasome by betulinic acid and its derivatives. | FEBS letters 20071016 |
| Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-o-(3',3'-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. | Antimicrobial agents and chemotherapy 20071001 |
| Glucuronidation of anti-HIV drug candidate bevirimat: identification of human UDP-glucuronosyltransferases and species differences. | Drug metabolism and disposition: the biological fate of chemicals 20070301 |
| New developments in natural products-based anti-AIDS research. | Medicinal research reviews 20070101 |
| Multiple-dose pharmacokinetics and safety of bevirimat, a novel inhibitor of HIV maturation, in healthy volunteers. | Clinical pharmacokinetics 20070101 |
| Maturation inhibitors: a new therapeutic class targets the virus structure. | AIDS reviews 20070101 |
| Potent activity of the HIV-1 maturation inhibitor bevirimat in SCID-hu Thy/Liv mice. | PloS one 20070101 |
| The 3-O-(3',3'-dimethylsuccinyl) derivative of betulinic acid (DSB) inhibits the assembly of virus-like particles in HIV-1 Gag precursor-expressing cells. | Antiviral therapy 20070101 |
| Determinants of activity of the HIV-1 maturation inhibitor PA-457. | Virology 20061201 |
| Human immunodeficiency virus type 1 resistance to the small molecule maturation inhibitor 3-O-(3',3'-dimethylsuccinyl)-betulinic acid is conferred by a variety of single amino acid substitutions at the CA-SP1 cleavage site in Gag. | Journal of virology 20061201 |
| In vitro resistance to the human immunodeficiency virus type 1 maturation inhibitor PA-457 (Bevirimat). | Journal of virology 20061101 |
| Anti-AIDS agents 69. Moronic acid and other triterpene derivatives as novel potent anti-HIV agents. | Journal of medicinal chemistry 20060907 |
| Structural characterization of anti-HIV drug candidate PA-457 [3-O-(3',3'-dimethylsuccinyl)-betulinic acid] and its acyl glucuronides in rat bile and evaluation of in vitro stability in human and animal liver microsomes and plasma. | Drug metabolism and disposition: the biological fate of chemicals 20060901 |
| Drug evaluation: bevirimat--HIV Gag protein and viral maturation inhibitor. | Current opinion in investigational drugs (London, England : 2000) 20060801 |
| A new assault on HIV. | Scientific American 20060601 |
| 3-O-(3',3'-dimethysuccinyl) betulinic acid inhibits maturation of the human immunodeficiency virus type 1 Gag precursor assembled in vitro. | Journal of virology 20060601 |
| Synthesis and anti-HIV activity of bi-functional betulinic acid derivatives. | Bioorganic & medicinal chemistry 20060401 |
| 3-O-Glutaryl-dihydrobetulin and related monoacyl derivatives as potent anti-HIV agents. | Bioorganic & medicinal chemistry letters 20041206 |
| Bifunctional anti-human immunodeficiency virus type 1 small molecules with two novel mechanisms of action. | Antimicrobial agents and chemotherapy 20040201 |
| Small-molecule inhibition of human immunodeficiency virus type 1 replication by specific targeting of the final step of virion maturation. | Journal of virology 20040101 |
| The sequence of the CA-SP1 junction accounts for the differential sensitivity of HIV-1 and SIV to the small molecule maturation inhibitor 3-O-{3',3'-dimethylsuccinyl}-betulinic acid. | Retrovirology 20040101 |
| PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. | Proceedings of the National Academy of Sciences of the United States of America 20031111 |
| Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. | Antimicrobial agents and chemotherapy 20010401 |
| 3,28-Di-O-(dimethylsuccinyl)-betulin isomers as anti-HIV agents. | Bioorganic & medicinal chemistry letters 20010122 |
| Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. | Journal of natural products 20001201 |
| Synthesis and anti-HIV activity of 3-alkylamido-3-deoxy-betulinic acid derivatives. | Chemical & pharmaceutical bulletin 20000901 |
| Anti-AIDS agents. 34. Synthesis and structure-activity relationships of betulin derivatives as anti-HIV agents. | Journal of medicinal chemistry 19981105 |
| Anti-AIDS agents--XXVII. Synthesis and anti-HIV activity of betulinic acid and dihydrobetulinic acid derivatives. | Bioorganic & medicinal chemistry 19971201 |
| Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. | Journal of medicinal chemistry 19960301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.