200,000+ products from a single source!
sales@angenechem.com
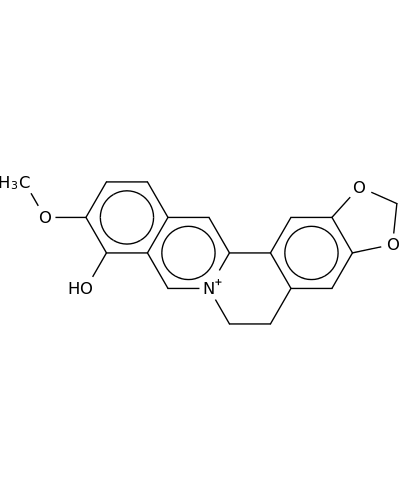
17388-19-1 | Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium, 5,6-dihydro-9-hydroxy-10-methoxy-
CAS No: 17388-19-1 Catalog No: AG001Z99 MDL No:
Product Description
Catalog Number:
AG001Z99
Chemical Name:
Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium, 5,6-dihydro-9-hydroxy-10-methoxy-
CAS Number:
17388-19-1
Molecular Formula:
C19H16NO4+
Molecular Weight:
322.3346
IUPAC Name:
17-methoxy-5,7-dioxa-13-azoniapentacyclo[11.8.0.02,10.04,8.015,20]henicosa-1(13),2,4(8),9,14,16,18,20-octaen-16-ol
InChI:
InChI=1S/C19H15NO4/c1-22-16-3-2-11-6-15-13-8-18-17(23-10-24-18)7-12(13)4-5-20(15)9-14(11)19(16)21/h2-3,6-9H,4-5,10H2,1H3/p+1
InChI Key:
GLYPKDKODVRYGP-UHFFFAOYSA-O
SMILES:
COc1ccc2c(c1O)c[n+]1c(c2)c2cc3OCOc3cc2CC1
Properties
Complexity:
474
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
322.108g/mol
Formal Charge:
1
Heavy Atom Count:
24
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
322.34g/mol
Monoisotopic Mass:
322.108g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
51.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.3
Literature
| Title | Journal |
|---|---|
| Structure-activity studies of some berberine analogs as inhibitors of Toxoplasma gondii. | Bioorganic & medicinal chemistry letters 20120415 |
| Synthesis of 9-substituted derivatives of berberine as anti-HIV agents. | European journal of medicinal chemistry 20110401 |
| Investigating the potential for toxicity from long-term use of the herbal products, goldenseal and milk thistle. | Toxicologic pathology 20110201 |
| Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. | Journal of translational medicine 20110101 |
| Design, synthesis, and cholesterol-lowering efficacy for prodrugs of berberrubine. | Bioorganic & medicinal chemistry 20100901 |
| New alkaloids from Corydalis species. | Natural product research 20090101 |
| [Absorption of coptisine chloride and berberrubine across human intestinal epithelial by using human Caco-2 cell monolayers]. | Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20071201 |
| Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. | Drug metabolism and disposition: the biological fate of chemicals 20061201 |
| Synthesis of 13-(substituted benzyl) berberine and berberrubine derivatives as antifungal agents. | Bioorganic & medicinal chemistry letters 20060801 |
| Effect of berberrubine on interleukin-8 and monocyte chemotactic protein-1 expression in human retinal pigment epithelial cell line. | Life sciences 20060801 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Synthesis and antihyperglycemic evaluation of various protoberberine derivatives. | Bioorganic & medicinal chemistry letters 20060301 |
| Antimicrobial activity of 9-O-acyl- and 9-O-alkylberberrubine derivatives. | Planta medica 20020301 |
| Antimicrobial activity of 9-O-acyl- and 9-O-benzoyl-substituted berberrubines. | Planta medica 20000501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.