200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 17241-59-7
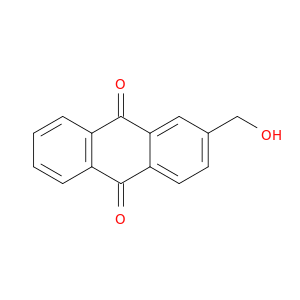
17241-59-7 | 2-(Hydroxymethyl)anthracene-9,10-dione
CAS No: 17241-59-7 Catalog No: AG003F2M MDL No:MFCD00001236
Product Description
Catalog Number:
AG003F2M
Chemical Name:
2-(Hydroxymethyl)anthracene-9,10-dione
CAS Number:
17241-59-7
Molecular Formula:
C15H10O3
Molecular Weight:
238.2381
MDL Number:
MFCD00001236
IUPAC Name:
2-(hydroxymethyl)anthracene-9,10-dione
InChI:
InChI=1S/C15H10O3/c16-8-9-5-6-12-13(7-9)15(18)11-4-2-1-3-10(11)14(12)17/h1-7,16H,8H2
InChI Key:
JYKHAJGLEVKEAA-UHFFFAOYSA-N
SMILES:
OCc1ccc2c(c1)C(=O)c1c(C2=O)cccc1
EC Number:
241-274-3
Properties
Complexity:
363
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
238.063g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
238.242g/mol
Monoisotopic Mass:
238.063g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
54.4A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
2.7
Literature
| Title | Journal |
|---|---|
| Cassia tora Linn Cream Inhibits Ultraviolet-B-Induced Psoriasis in Rats. | ISRN dermatology 20120101 |
| Exploitation of unique properties of zeolites in the development of gas sensors. | Sensors (Basel, Switzerland) 20120101 |
| Long-range intramolecular photoredox reaction via coupled charge and proton transfer of triplet excited anthraquinones mediated by water. | Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 20091001 |
| Formal intramolecular photoredox chemistry of anthraquinones in aqueous solution: photodeprotection for alcohols, aldehydes and ketones. | Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 20080501 |
| Plasma, tissue and urinary levels of aloin in rats after the administration of pure aloin. | Nutrition research and practice 20080101 |
| Chemical Constituents from the Stems of Morinda citrifolia Linn. | Archives of pharmacal research 20070701 |
| A simple and smart oxygen sensor based on the intrazeolite reactions of a substituted anthraquinone. | Chemical communications (Cambridge, England) 20061113 |
| Antibacterial activity of Tabebuia impetiginosa Martius ex DC (Taheebo) against Helicobacter pylori. | Journal of ethnopharmacology 20060421 |
| [Studies on the chemical constituents from stem of Chirita longgangensis var. Hongyao]. | Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 20060201 |
| Excited state intramolecular redox reaction of 2-(hydroxymethyl)anthraquinone in aqueous solution. | Chemical communications (Cambridge, England) 20020121 |
| Metabolism of 1,8-dihydroxy 3-hydroxy methyl anthraquinone (aloe-emodin) isolated from the leaves of Cassia tora in albino rats. | Phytotherapy research : PTR 20010801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.