200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 16875-10-8
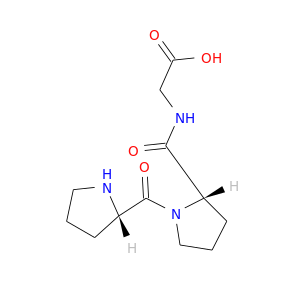
16875-10-8 | Glycine, L-prolyl-L-prolyl-
CAS No: 16875-10-8 Catalog No: AG001Y9J MDL No:
Product Description
Catalog Number:
AG001Y9J
Chemical Name:
Glycine, L-prolyl-L-prolyl-
CAS Number:
16875-10-8
Molecular Formula:
C12H19N3O4
Molecular Weight:
269.2970
IUPAC Name:
2-[[(2S)-1-[(2S)-pyrrolidine-2-carbonyl]pyrrolidine-2-carbonyl]amino]acetic acid
InChI:
InChI=1S/C12H19N3O4/c16-10(17)7-14-11(18)9-4-2-6-15(9)12(19)8-3-1-5-13-8/h8-9,13H,1-7H2,(H,14,18)(H,16,17)/t8-,9-/m0/s1
InChI Key:
LEIKGVHQTKHOLM-IUCAKERBSA-N
SMILES:
OC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1
Properties
Complexity:
385
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
269.138g/mol
Formal Charge:
0
Heavy Atom Count:
19
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
269.301g/mol
Monoisotopic Mass:
269.138g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
98.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-3
Literature
| Title | Journal |
|---|---|
| Temperature-dependent higher order structures of the (Pro-Pro-Gly)₁₀-modified dendrimer. | Biopolymers 20110401 |
| Semax and Pro-Gly-Pro activate the transcription of neurotrophins and their receptor genes after cerebral ischemia. | Cellular and molecular neurobiology 20100101 |
| The effect of deuterium oxide on the stability of the collagen model peptides H-(Pro-Pro-Gly)(10)-OH, H-(Gly-Pro-4(R)Hyp)(9)-OH, and Type I collagen. | Biopolymers 20100101 |
| Catalytic removal of N-allyloxycarbonyl groups using the [CpRu(IV)(pi-C3H5)(2-quinolinecarboxylato)]PF6 complex. A new efficient deprotecting method in peptide synthesis. | The Journal of organic chemistry 20060609 |
| Conformation of alloHyp in the Y position in the host-guest peptide with the pro-pro-gly sequence: implication of the destabilization of (Pro-alloHyp-Gly)10. | Biopolymers 20060215 |
| Completely geometrically optimized DFT/ONIOM triple-helical collagen-like structures containing the ProProGly, ProProAla, ProProDAla, and ProProDSer triads. | Journal of the American Chemical Society 20051019 |
| Collagen-like triple helix formation of synthetic (Pro-Pro-Gly)10 analogues: (4(S)-hydroxyprolyl-4(R)-hydroxyprolyl-Gly)10, (4(R)-hydroxyprolyl-4(R)-hydroxyprolyl-Gly)10 and (4(S)-fluoroprolyl-4(R)-fluoroprolyl-Gly)10. | Journal of peptide science : an official publication of the European Peptide Society 20051001 |
| Repetitive interactions observed in the crystal structure of a collagen-model peptide, [(Pro-Pro-Gly)9]3. | Journal of biochemistry 20050801 |
| Unexpected puckering of hydroxyproline in the guest triplets, hyp-pro-gly and pro-allohyp-gly sandwiched between pro-pro-gly sequence. | Chembiochem : a European journal of chemical biology 20050701 |
| Studies of the collagen-like peptide (Pro-Pro-Gly)(10) confirm that the shape and position of the type I collagen denaturation endotherm is governed by the rate of helix unfolding. | Journal of molecular biology 20040402 |
| Asymmetry in the triple helix of collagen-like heterotrimers confirms that external bonds stabilize collagen structure. | Journal of molecular biology 20030523 |
| Understanding the role of stereoelectronic effects in determining collagen stability. 2. A quantum mechanical/molecular mechanical study of (Proline-Proline-Glycine)(n) polypeptides. | Journal of the American Chemical Society 20020703 |
| Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)(10)](3). | Protein science : a publication of the Protein Society 20020201 |
| Adhesion of N-methacryloyl-omega-amino acid primers to collagen analyzed by 13C NMR. | Journal of dental research 20010301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.