200,000+ products from a single source!
sales@angenechem.com
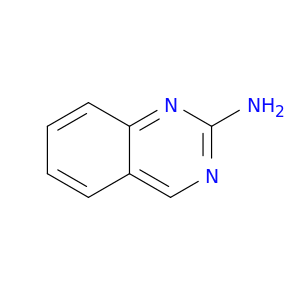
1687-51-0 | 2-Aminoquinazoline
CAS No: 1687-51-0 Catalog No: AG001Y8D MDL No:MFCD00956186
Product Description
Catalog Number:
AG001Y8D
Chemical Name:
2-Aminoquinazoline
CAS Number:
1687-51-0
Molecular Formula:
C8H7N3
Molecular Weight:
145.1613
MDL Number:
MFCD00956186
IUPAC Name:
quinazolin-2-amine
InChI:
InChI=1S/C8H7N3/c9-8-10-5-6-3-1-2-4-7(6)11-8/h1-5H,(H2,9,10,11)
InChI Key:
CZAAKPFIWJXPQT-UHFFFAOYSA-N
SMILES:
Nc1ncc2c(n1)cccc2
Properties
Complexity:
137
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
145.064g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
145.165g/mol
Monoisotopic Mass:
145.064g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
51.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.3
Literature
| Title | Journal |
|---|---|
| An aminoquinazoline inhibitor of the essential bacterial cell wall synthetic enzyme GlmU has a unique non-protein-kinase-like binding mode. | The Biochemical journal 20120915 |
| Substituted aminopyrimidine protein kinase B (PknB) inhibitors show activity against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20120501 |
| Discovery of novel aminoquinazolin-7-yl 6,7-dihydro-indol-4-ones as potent, selective inhibitors of heat shock protein 90. | Bioorganic & medicinal chemistry letters 20120401 |
| Synthesis of aminoquinazoline derivatives and their antiproliferative activities against melanoma cell line. | Bioorganic & medicinal chemistry letters 20101001 |
| Anticonvulsant Activity of Schiff Bases of 3-Amino-6,8-dibromo-2-phenyl-quinazolin-4(3H)-ones. | Indian journal of pharmaceutical sciences 20100101 |
| 2-(5,6-Di-hydro-benzimidazolo[1,2-c]quinazolin-6-yl)aniline methanol solvate. | Acta crystallographica. Section E, Structure reports online 20090701 |
| Modulation of erlotinib pharmacokinetics in mice by a novel cytochrome P450 3A4 inhibitor, BAS 100. | British journal of cancer 20080520 |
| Structure-guided design of aminopyrimidine amides as potent, selective inhibitors of lymphocyte specific kinase: synthesis, structure-activity relationships, and inhibition of in vivo T cell activation. | Journal of medicinal chemistry 20080327 |
| From arylureas to biarylamides to aminoquinazolines: discovery of a novel, potent TRPV1 antagonist. | Bioorganic & medicinal chemistry letters 20061001 |
| Discovery of aminoquinazolines as potent, orally bioavailable inhibitors of Lck: synthesis, SAR, and in vivo anti-inflammatory activity. | Journal of medicinal chemistry 20060921 |
| The combi-targeting concept: synthesis of stable nitrosoureas designed to inhibit the epidermal growth factor receptor (EGFR). | Journal of medicinal chemistry 20060615 |
| Nitrenes, diradicals, and ylides. Ring expansion and ring opening in 2-quinazolylnitrenes. | The Journal of organic chemistry 20060526 |
| 2-Aminoquinazoline inhibitors of cyclin-dependent kinases. | Bioorganic & medicinal chemistry letters 20050901 |
| Multiple mechanisms of action of ZR2002 in human breast cancer cells: a novel combi-molecule designed to block signaling mediated by the ERB family of oncogenes and to damage genomic DNA. | International journal of cancer 20041110 |
| Identification and specificity studies of small-molecule ligands for SH3 protein domains. | Journal of medicinal chemistry 20041021 |
| Characterization of inhibitor binding sites of mitochondrial complex I using fluorescent inhibitor. | Biochimica et biophysica acta 20030818 |
| Design of triple helix forming C-glycoside molecules. | Journal of the American Chemical Society 20030226 |
| Physical methods to determine the binding mode of putative ligands for hepatitis C virus NS3 helicase. | Analytical biochemistry 20021015 |
Related Products
© 2019 Angene International Limited. All rights Reserved.