200,000+ products from a single source!
sales@angenechem.com
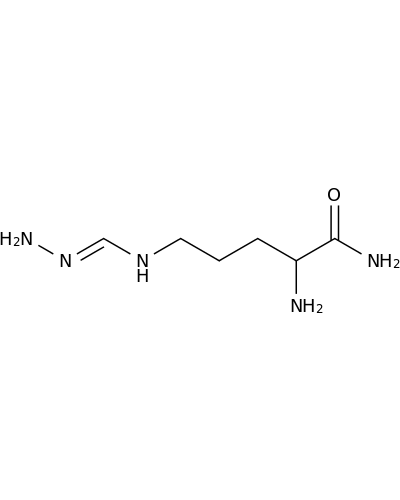
16709-23-2 | Pentanamide, 2-amino-5-[(aminoiminomethyl)amino]-
CAS No: 16709-23-2 Catalog No: AG001X43 MDL No:
Product Description
Catalog Number:
AG001X43
Chemical Name:
Pentanamide, 2-amino-5-[(aminoiminomethyl)amino]-
CAS Number:
16709-23-2
Molecular Formula:
C6H15N5O
Molecular Weight:
173.2162
IUPAC Name:
2-amino-5-(diaminomethylideneamino)pentanamide
InChI:
InChI=1S/C6H15N5O/c7-4(5(8)12)2-1-3-11-6(9)10/h4H,1-3,7H2,(H2,8,12)(H4,9,10,11)
InChI Key:
ULEBESPCVWBNIF-UHFFFAOYSA-N
SMILES:
NN=CNCCCC(C(=O)N)N
NSC Number:
203804
Properties
Complexity:
175
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
173.128g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
173.22g/mol
Monoisotopic Mass:
173.128g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
134A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.6
Literature
| Title | Journal |
|---|---|
| Molecular dynamics simulation of the induced-fit binding process of DNA aptamer and L-argininamide. | Biotechnology journal 20121101 |
| Sided functions of an arginine-agmatine antiporter oriented in liposomes. | Biochemistry 20120227 |
| Dual-polarization interferometry for quantification of small molecules using aptamers. | Analytical and bioanalytical chemistry 20120101 |
| Application of the guanidine-acylguanidine bioisosteric approach to argininamide-type NPY Y₂ receptor antagonists. | ChemMedChem 20110905 |
| [³H]UR-MK136: a highly potent and selective radioligand for neuropeptide Y Y₁ receptors. | ChemMedChem 20110905 |
| Design of aptamer-based sensing platform using triple-helix molecular switch. | Analytical chemistry 20110901 |
| Fluorescent aptasensors based on conformational adaptability of abasic site-containing aptamers in combination with abasic site-binding ligands. | Biosensors & bioelectronics 20110815 |
| Red-fluorescent argininamide-type NPY Y1 receptor antagonists as pharmacological tools. | Bioorganic & medicinal chemistry 20110501 |
| Label-free aptamer-based sensors for L-argininamide by using nucleic acid minor groove binding dyes. | Chemical communications (Cambridge, England) 20110321 |
| N(G)-Acyl-argininamides as NPY Y(1) receptor antagonists: Influence of structurally diverse acyl substituents on stability and affinity. | Bioorganic & medicinal chemistry 20100901 |
| RNA dynamics by design: biasing ensembles towards the ligand-bound state. | Angewandte Chemie (International ed. in English) 20100802 |
| Bivalent argininamide-type neuropeptide y y(1) antagonists do not support the hypothesis of receptor dimerisation. | ChemMedChem 20091001 |
| Acridone-labeled DNA aptamer for the detection of biomolecules. | Nucleic acids symposium series (2004) 20090101 |
| Guanidine-acylguanidine bioisosteric approach in the design of radioligands: synthesis of a tritium-labeled N(G)-propionylargininamide ([3H]-UR-MK114) as a highly potent and selective neuropeptide Y Y1 receptor antagonist. | Journal of medicinal chemistry 20081225 |
| Single-molecule study of the inhibition of HIV-1 transactivation response region DNA/DNA annealing by argininamide. | Journal of the American Chemical Society 20070822 |
| CE with electrochemical detection for investigation of label-free recognition of amino acid amides by guanine-rich DNA aptamers. | Electrophoresis 20070801 |
| L-argininamide improves the refolding more effectively than L-arginine. | Journal of biotechnology 20070615 |
| Energetic basis of molecular recognition in a DNA aptamer. | Biophysical chemistry 20070301 |
| Adaptive recognition of small molecules by nucleic acid aptamers through a label-free approach. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| Structural features of the L-argininamide-binding DNA aptamer studied with ESI-FTMS. | Analytical chemistry 20061015 |
| Biomolecular sensor based on fluorescence-labeled aptamer. | Bioorganic & medicinal chemistry letters 20060815 |
| Resolving the motional modes that code for RNA adaptation. | Science (New York, N.Y.) 20060203 |
| The effect of force on thermodynamics and kinetics: unfolding single RNA molecules. | Biochemical Society transactions 20041101 |
| Ligand-induced changes in 2-aminopurine fluorescence as a probe for small molecule binding to HIV-1 TAR RNA. | RNA (New York, N.Y.) 20040901 |
| DNA helix-stack switching as the basis for the design of versatile deoxyribosensors. | Journal of molecular biology 20040709 |
| Argininamide binding arrests global motions in HIV-1 TAR RNA: comparison with Mg2+-induced conformational stabilization. | Journal of molecular biology 20040416 |
| Rational design of inhibitors of HIV-1 TAR RNA through the stabilisation of electrostatic 'hot spots'. | Journal of molecular biology 20040213 |
| Structural basis of the highly efficient trapping of the HIV Tat protein by an RNA aptamer. | Structure (London, England : 1993) 20030501 |
| Investigation of RNA-protein and RNA-metal ion interactions by electron paramagnetic resonance spectroscopy. The HIV TAR-Tat motif. | Chemistry & biology 20020601 |
| Base flexibility in HIV-2 TAR RNA mapped by solution (15)N, (13)C NMR relaxation. | Journal of molecular biology 20020322 |
| The specific hydrolysis of HIV-1 TAR RNA element with the anti-TAR hammerhead ribozyme: structural and functional implications. | International journal of biological macromolecules 20010612 |
Related Products
© 2019 Angene International Limited. All rights Reserved.