200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 1670-84-4
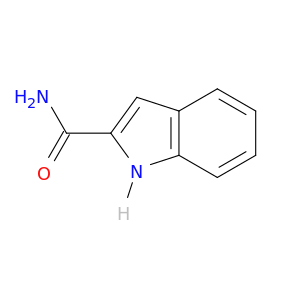
1670-84-4 | Indole-2-carboxamide
CAS No: 1670-84-4 Catalog No: AG00AD3C MDL No:MFCD00231036
Product Description
Catalog Number:
AG00AD3C
Chemical Name:
Indole-2-carboxamide
CAS Number:
1670-84-4
Molecular Formula:
C9H8N2O
Molecular Weight:
160.1726
MDL Number:
MFCD00231036
IUPAC Name:
1H-indole-2-carboxamide
InChI:
InChI=1S/C9H8N2O/c10-9(12)8-5-6-3-1-2-4-7(6)11-8/h1-5,11H,(H2,10,12)
InChI Key:
VFHUJFBEFDVZPJ-UHFFFAOYSA-N
SMILES:
NC(=O)c1cc2c([nH]1)cccc2
EC Number:
605-465-9
Properties
Complexity:
193
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
160.064g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
160.176g/mol
Monoisotopic Mass:
160.064g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
58.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| New nitrogen containing substituents at the indole-2-carboxamide yield high potent and broad spectrum indolylarylsulfone HIV-1 non-nucleoside reverse transcriptase inhibitors. | Journal of medicinal chemistry 20120726 |
| Progress in structure based drug design for G protein-coupled receptors. | Journal of medicinal chemistry 20110714 |
| Pharmacokinetic optimisation of novel indole-2-carboxamide cannabinoid CB1 antagonists. | Bioorganic & medicinal chemistry letters 20110401 |
| Indolylarylsulfones as HIV-1 non-nucleoside reverse transcriptase inhibitors: new cyclic substituents at indole-2-carboxamide. | Journal of medicinal chemistry 20110324 |
| The discovery of novel indole-2-carboxamides as cannabinoid CB(1) receptor antagonists. | Bioorganic & medicinal chemistry letters 20110101 |
| Indole-2-amide based biochemical antagonist of Dishevelled PDZ domain interaction down-regulates Dishevelled-driven Tcf transcriptional activity. | Bioorganic & medicinal chemistry letters 20080201 |
| Design, molecular modeling, synthesis, and anti-HIV-1 activity of new indolyl aryl sulfones. Novel derivatives of the indole-2-carboxamide. | Journal of medicinal chemistry 20060601 |
| Indolyl aryl sulphones as HIV-1 non-nucleoside reverse transcriptase inhibitors: synthesis, biological evaluation and binding mode studies of new derivatives at indole-2-carboxamide. | Antiviral chemistry & chemotherapy 20060101 |
| CoMFA and docking studies on glycogen phosphorylase a inhibitors as antidiabetic agents. | Journal of chemical information and modeling 20050101 |
| Inhibitory mode of indole-2-carboxamide derivatives against HLGPa: molecular docking and 3D-QSAR analyses. | Bioorganic & medicinal chemistry 20040801 |
| Renaissance of NMDA receptor antagonists: do they have a role in the pharmacotherapy for alcoholism? | IDrugs : the investigational drugs journal 20040401 |
| NR2B subunit selective NMDA antagonists inhibit neurotoxic effect of alcohol-withdrawal in primary cultures of rat cortical neurones. | Neurochemistry international 20040101 |
| Indole-2-carboxamides as novel NR2B selective NMDA receptor antagonists. | Bioorganic & medicinal chemistry letters 20031103 |
| Indole amide hydroxamic acids as potent inhibitors of histone deacetylases. | Bioorganic & medicinal chemistry letters 20030602 |
| Diverse effects of two allosteric inhibitors on the phosphorylation state of glycogen phosphorylase in hepatocytes. | The Biochemical journal 20021115 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.