200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1633-22-3
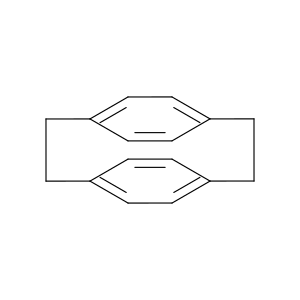
1633-22-3 | Tricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene
CAS No: 1633-22-3 Catalog No: AG001U0I MDL No:MFCD00003707
Product Description
Catalog Number:
AG001U0I
Chemical Name:
Tricyclo[8.2.2.24,7]hexadeca-4,6,10,12,13,15-hexaene
CAS Number:
1633-22-3
Molecular Formula:
C16H16
Molecular Weight:
208.2982
MDL Number:
MFCD00003707
IUPAC Name:
tricyclo[8.2.2.24,7]hexadeca-1(13),4,6,10(14),11,15-hexaene
InChI:
InChI=1S/C16H16/c1-2-14-4-3-13(1)9-10-15-5-7-16(8-6-15)12-11-14/h1-8H,9-12H2
InChI Key:
OOLUVSIJOMLOCB-UHFFFAOYSA-N
SMILES:
C1Cc2ccc(cc2)CCc2ccc1cc2
EC Number:
216-644-2
UNII:
0I9PQB142O
NSC Number:
91575
Properties
Complexity:
156
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
208.125g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
208.304g/mol
Monoisotopic Mass:
208.125g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.7
Literature
| Title | Journal |
|---|---|
| A pass too far: dissociation of internal energy selected paracyclophane cations, theory and experiment. | Physical chemistry chemical physics : PCCP 20120914 |
| Spectroelectrochemistry of a photochromic [2.2]paracyclophane-bridged imidazole dimer: clarification of the electrochemical behavior of HABI. | The journal of physical chemistry. A 20120628 |
| Photochromic properties of [2.2]paracyclophane-bridged imidazole dimer with increased photosensitivity by introducing pyrenyl moiety. | The journal of physical chemistry. A 20111124 |
| Comprehensive understanding of structure- photosensitivity relationships of photochromic [2.2]paracyclophane-bridged imidazole dimers. | The journal of physical chemistry. A 20110512 |
| 12,12'-[2,2'-Oxybis(ethane-2,1-di-yl)bis-(-oxy)]bis-[(R(p))-4-bromo-[2.2]paracyclo-phane]. | Acta crystallographica. Section E, Structure reports online 20110401 |
| Single-molecule conductance through multiple π-π-stacked benzene rings determined with direct electrode-to-benzene ring connections. | Journal of the American Chemical Society 20110223 |
| Facile synthesis of planar chiral N-oxides and their use in Lewis base catalysis. | Chemical communications (Cambridge, England) 20110107 |
| Synthesis, structure, and electronic and photophysical properties of two- and three-layered [3.3]paracyclophane-based donor-acceptor systems. | The Journal of organic chemistry 20100917 |
| Multitiered 2D pi-stacked conjugated polymers based on pseudo-geminal disubstituted [2.2]paracyclophane. | Journal of the American Chemical Society 20100908 |
| 5,8-Dibromo-14,15,17,18-tetra-methyl-2,11-dithia-[3.3]paracyclo-phane. | Acta crystallographica. Section E, Structure reports online 20100801 |
| EPR and ENDOR studies of dimeric paracyclophane radical cations and dications containing tri- and pentamethylene-bridged p-phenylene diamine units. | The journal of physical chemistry. A 20100617 |
| A fast photochromic molecule that colors only under UV light. | Journal of the American Chemical Society 20090401 |
| 4-Amino-13-(1-naphth-yl)-[2,2]paracyclo-phane. | Acta crystallographica. Section E, Structure reports online 20080401 |
| Symmetrically tetrasubstituted [2.2]paracyclophanes: their systematization and regioselective synthesis of several types of bis-bifunctional derivatives by double electrophilic substitution. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| The 'cyclophene' [2.2.2](1,2,4)cyclophan-9-ene. | Acta crystallographica. Section C, Crystal structure communications 20071201 |
| Designing cyclophane-based molecular wire sensors. | The journal of physical chemistry. B 20061130 |
| Fancy bioisosteres: novel paracyclophane derivatives as super-affinity dopamine D3 receptor antagonists. | Journal of medicinal chemistry 20060615 |
| Role of electron density and magnetic couplings on the nucleus-independent chemical shift (NICS) profiles of [2.2]paracyclophane and related species. | The Journal of organic chemistry 20060217 |
| Design and construction of a 2D metal organic framework with multiple cavities: a nonregular net with a paracyclophane that codes for multiply fused nodes. | Journal of the American Chemical Society 20051019 |
| Theoretical study of the effects of solvent environment on photophysical properties and electronic structure of paracyclophane chromophores. | The Journal of chemical physics 20050608 |
| Synthesis of macrocycles via cobalt-mediated [2 + 2 + 2] cycloadditions. | Journal of the American Chemical Society 20050316 |
| Atropisomeric (R,R)-2,2'-Bi([2]paracyclo[2](5,8)quinolinophane) and (R,R)-1,1'-Bi([2]paracyclo[2](5,8)isoquinolinophane): synthesis, structural analysis, and chiroptical properties. | The Journal of organic chemistry 20050204 |
| Two-photon absorption in three-dimensional chromophores based on [2.2]-paracyclophane. | Journal of the American Chemical Society 20040922 |
| Electrophilic substitution of dibromoparacyclophane: a route to novel paracyclophane phosphine ligands. | Organic letters 20040610 |
| Binuclear rhenium(I) complexes with bridging [2.2]paracyclophane-diimine ligands: probing electronic coupling through pi-pi interactions. | Inorganic chemistry 20040126 |
| Through-space delocalized water-soluble paracyclophane bichromophores: new fluorescent optical reporters. | Chemistry (Weinheim an der Bergstrasse, Germany) 20030721 |
| Three-dimensional nonlinear optical chromophores based on through-space delocalization. | Journal of the American Chemical Society 20021113 |
| Water-soluble oligomer dimers based on paracyclophane: a new optical platform for fluorescent sensor applications. | Journal of the American Chemical Society 20021009 |
| Phosphonites based on the paracyclophane backbone: new ligands for highly selective rhodium-catalyzed asymmetric hydrogenation. | Organic letters 20011115 |
| Anti-inflammatory planar chiral [2.2]paracyclophaneacetic acid enantiomers. | Inflammation research : official journal of the European Histamine Research Society ... [et al.] 20010701 |
Related Products
© 2019 Angene International Limited. All rights Reserved.