200,000+ products from a single source!
sales@angenechem.com
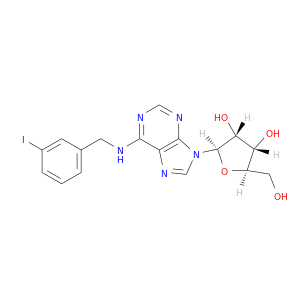
163152-30-5 | Adenosine, N-[(3-iodophenyl)methyl]-
CAS No: 163152-30-5 Catalog No: AG001TTI MDL No:
Product Description
Catalog Number:
AG001TTI
Chemical Name:
Adenosine, N-[(3-iodophenyl)methyl]-
CAS Number:
163152-30-5
Molecular Formula:
C17H18IN5O4
Molecular Weight:
483.2604
IUPAC Name:
(2R,3S,4R,5R)-2-(hydroxymethyl)-5-[6-[(3-iodophenyl)methylamino]purin-9-yl]oxolane-3,4-diol
InChI:
InChI=1S/C17H18IN5O4/c18-10-3-1-2-9(4-10)5-19-15-12-16(21-7-20-15)23(8-22-12)17-14(26)13(25)11(6-24)27-17/h1-4,7-8,11,13-14,17,24-26H,5-6H2,(H,19,20,21)/t11-,13-,14-,17-/m1/s1
InChI Key:
TWLWIJNPUVZDOB-LSCFUAHRSA-N
SMILES:
OC[C@H]1O[C@H]([C@@H]([C@@H]1O)O)n1cnc2c1ncnc2NCc1cccc(c1)I
Properties
Complexity:
506
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
483.04g/mol
Formal Charge:
0
Heavy Atom Count:
27
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
483.266g/mol
Monoisotopic Mass:
483.04g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
126A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.8
Literature
| Title | Journal |
|---|---|
| Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. | Bioorganic & medicinal chemistry 20070601 |
| (N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists. | Journal of medicinal chemistry 20050324 |
| Synthesis, biological evaluation, and molecular modeling of ribose-modified adenosine analogues as adenosine receptor agonists. | Journal of medicinal chemistry 20050310 |
| Structural determinants of A(3) adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary. | Journal of medicinal chemistry 20020926 |
| 5'-O-alkyl ethers of N,2-substituted adenosine derivatives: partial agonists for the adenosine A1 and A3 receptors. | Journal of medicinal chemistry 20010830 |
| 2'-C-Methyl analogues of selective adenosine receptor agonists: synthesis and binding studies. | Journal of medicinal chemistry 19980507 |
| 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. | Journal of medicinal chemistry 19941014 |
Related Products
© 2019 Angene International Limited. All rights Reserved.