200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 16312-79-1
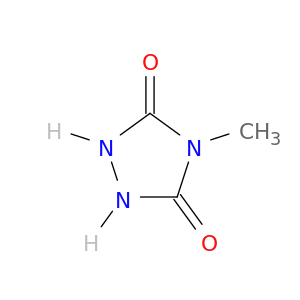
16312-79-1 | 1,2,4-Triazolidine-3,5-dione, 4-methyl-
CAS No: 16312-79-1 Catalog No: AG001TS7 MDL No:MFCD00005227
Product Description
Catalog Number:
AG001TS7
Chemical Name:
1,2,4-Triazolidine-3,5-dione, 4-methyl-
CAS Number:
16312-79-1
Molecular Formula:
C3H5N3O2
Molecular Weight:
115.0907
MDL Number:
MFCD00005227
IUPAC Name:
4-methyl-1,2,4-triazolidine-3,5-dione
InChI:
InChI=1S/C3H5N3O2/c1-6-2(7)4-5-3(6)8/h1H3,(H,4,7)(H,5,8)
InChI Key:
XJOOWWMZMXBLAO-UHFFFAOYSA-N
SMILES:
O=c1[nH][nH]c(=O)n1C
Properties
Complexity:
128
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
115.038g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
115.092g/mol
Monoisotopic Mass:
115.038g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
61.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.9
Literature
| Title | Journal |
|---|---|
| Identification and behavioral evaluation of sex pheromone components of the Chinese pine caterpillar moth, Dendrolimus tabulaeformis. | PloS one 20120101 |
| Identification and field evaluation of sex pheromone components of the pear barkminer moth, Spulerina astaurota. | Journal of chemical ecology 20111101 |
| Improved HRGC separation of cis, trans CLA isomers as Diels-Alder adducts of alkyl esters. | Journal of chromatographic science 20110501 |
| Vibrational spectra and structures of the anions of urazole and 4-methylurazole: DFT calculations of the normal modes and the influence of hydrogen bonding. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20110301 |
| Integrated genomics of ovarian xenograft tumor progression and chemotherapy response. | BMC cancer 20110101 |
| Major sex pheromone components of the Australian gum leaf skeletonizer Uraba lugens: (10E,12Z)-hexadecadien-1-yl acetate and (10E,12Z)-hexadecadien-1-ol. | Journal of chemical ecology 20080901 |
| Natural products as an inspiration in the diversity-oriented synthesis of bioactive compound libraries. | Natural product reports 20080801 |
| Key biosynthetic gene subfamily recruited for pheromone production prior to the extensive radiation of Lepidoptera. | BMC evolutionary biology 20080101 |
| (11Z,13E)-Hexadecadien-1-yl acetate: sex pheromone of the grass webworm Herpetogramma licarsisalis-identification, synthesis, and field bioassays. | Journal of chemical ecology 20070401 |
| Partial elucidation of Trichogramma putative sex pheromone at trace levels by solid-phase microextraction and gas chromatography-mass spectrometry studies. | Journal of chromatography. A 20050304 |
| Efficient method to locate double bond positions in conjugated trienes. | Journal of chromatography. A 20040903 |
| [Determination of double bond position in conjugated dienes in sex pheromones of Dendrolimus spp]. | Se pu = Chinese journal of chromatography 20040301 |
| The first method for protection-deprotection of the indole 2,3-pi bond. | Organic letters 20030529 |
| Low-temperature photosensitized oxidation of a guanosine derivative and formation of an imidazole ring-opened product. | Journal of the American Chemical Society 20020417 |
| Stereochemistry in the reaction of 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) with beta,beta dimethyl-p-methoxystyrene. Are open biradicals the reaction intermediates? | The Journal of organic chemistry 20010601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







