200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1627-73-2
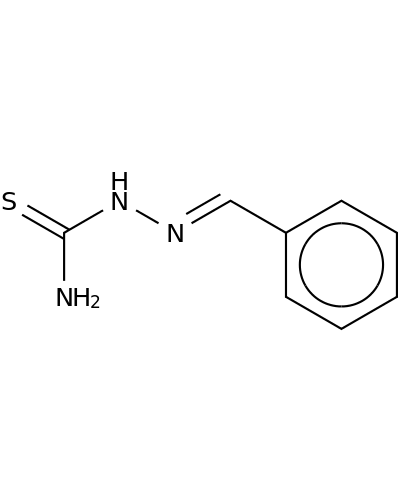
1627-73-2 | Hydrazinecarbothioamide, 2-(phenylmethylene)-
CAS No: 1627-73-2 Catalog No: AG001TCG MDL No:MFCD00084893
Product Description
Catalog Number:
AG001TCG
Chemical Name:
Hydrazinecarbothioamide, 2-(phenylmethylene)-
CAS Number:
1627-73-2
Molecular Formula:
C8H9N3S
Molecular Weight:
179.2422
MDL Number:
MFCD00084893
IUPAC Name:
[(E)-benzylideneamino]thiourea
InChI:
InChI=1S/C8H9N3S/c9-8(12)11-10-6-7-4-2-1-3-5-7/h1-6H,(H3,9,11,12)/b10-6+
InChI Key:
UYHCMAZIKNVDSX-UXBLZVDNSA-N
SMILES:
NC(=S)NN=Cc1ccccc1
EC Number:
216-618-0
UNII:
CTH1TK4P2V
NSC Number:
267
Properties
Complexity:
173
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
179.052g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
179.241g/mol
Monoisotopic Mass:
179.052g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
82.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.9
Literature
| Title | Journal |
|---|---|
| The synthesis and antiparasitic activity of aryl- and ferrocenyl-derived thiosemicarbazone ruthenium(II)-arene complexes. | Dalton transactions (Cambridge, England : 2003) 20130407 |
| Structural characteristics of thiosemicarbazones as inhibitors of melanogenesis. | Bioorganic & medicinal chemistry letters 20101115 |
| Structural requirement(s) of N-phenylthioureas and benzaldehyde thiosemicarbazones as inhibitors of melanogenesis in melanoma B 16 cells. | Bioorganic & medicinal chemistry letters 20100501 |
| 3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors. | Bioorganic & medicinal chemistry 20070301 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Three-dimensional quantitative structure-activity relationship analysis of a set of Plasmodium falciparum dihydrofolate reductase inhibitors using a pharmacophore generation approach. | Journal of medicinal chemistry 20040812 |
| Docking and database screening reveal new classes of Plasmodium falciparum dihydrofolate reductase inhibitors. | Journal of medicinal chemistry 20030703 |
Related Products
© 2019 Angene International Limited. All rights Reserved.