200,000+ products from a single source!
sales@angenechem.com
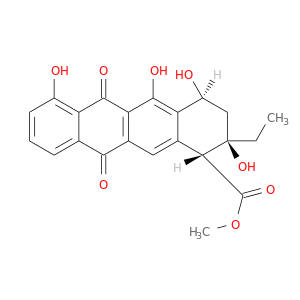
16234-96-1 | 1-Naphthacenecarboxylic acid, 2-ethyl-1,2,3,4,6,11-hexahydro-2,4,5,7-tetrahydroxy-6,11-dioxo-, methyl ester, (1R,2R,4S)-
CAS No: 16234-96-1 Catalog No: AG001T1E MDL No:
Product Description
Catalog Number:
AG001T1E
Chemical Name:
1-Naphthacenecarboxylic acid, 2-ethyl-1,2,3,4,6,11-hexahydro-2,4,5,7-tetrahydroxy-6,11-dioxo-, methyl ester, (1R,2R,4S)-
CAS Number:
16234-96-1
Molecular Formula:
C22H20O8
Molecular Weight:
412.3894
IUPAC Name:
methyl (1R,2R,4S)-2-ethyl-2,4,5,7-tetrahydroxy-6,11-dioxo-3,4-dihydro-1H-tetracene-1-carboxylate
InChI:
InChI=1S/C22H20O8/c1-3-22(29)8-13(24)15-10(17(22)21(28)30-2)7-11-16(20(15)27)19(26)14-9(18(11)25)5-4-6-12(14)23/h4-7,13,17,23-24,27,29H,3,8H2,1-2H3/t13-,17-,22+/m0/s1
InChI Key:
RACGRCLGVYXIAO-YOKWENHESA-N
SMILES:
COC(=O)[C@@H]1c2cc3C(=O)c4cccc(c4C(=O)c3c(c2[C@H](C[C@]1(O)CC)O)O)O
Properties
Complexity:
737
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
412.116g/mol
Formal Charge:
0
Heavy Atom Count:
30
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
412.394g/mol
Monoisotopic Mass:
412.116g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
141A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.5
Literature
| Title | Journal |
|---|---|
| F420H2-dependent degradation of aflatoxin and other furanocoumarins is widespread throughout the actinomycetales. | PloS one 20120101 |
| Tautomerism in 11-hydroxyaklavinone: a DFT study. | TheScientificWorldJournal 20120101 |
| Identification and characterization of two families of F420 H2-dependent reductases from Mycobacteria that catalyse aflatoxin degradation. | Molecular microbiology 20101101 |
| Structural basis for substrate recognition and specificity in aklavinone-11-hydroxylase from rhodomycin biosynthesis. | Journal of molecular biology 20091106 |
| Predicted roles of the uncharacterized clustered genes in aflatoxin biosynthesis. | Toxins 20090901 |
| Protein functional surfaces: global shape matching and local spatial alignments of ligand binding sites. | BMC structural biology 20080101 |
| AknT is an activating protein for the glycosyltransferase AknS in L-aminodeoxysugar transfer to the aglycone of aclacinomycin A. | Chemistry & biology 20050501 |
| Blanchaquinone: a new anthraquinone from an Australian Streptomyces sp. | Journal of natural products 20041001 |
| AknK is an L-2-deoxyfucosyltransferase in the biosynthesis of the anthracycline aclacinomycin A. | Biochemistry 20040420 |
| Crystal structure of aclacinomycin methylesterase with bound product analogues: implications for anthracycline recognition and mechanism. | The Journal of biological chemistry 20031003 |
| Expression, purification, and characterization of AknX anthrone oxygenase, which is involved in aklavinone biosynthesis in Streptomyces galilaeus. | Journal of bacteriology 20021101 |
| Characterization of mutations in aclacinomycin A-non-producing Streptomyces galilaeus strains with altered glycosylation patterns. | Microbiology (Reading, England) 20021101 |
| Cloning and characterization of Streptomyces galilaeus aclacinomycins polyketide synthase (PKS) cluster. | Gene 20020626 |
| Modification of aklavinone and aclacinomycins in vitro and in vivo by rhodomycin biosynthesis gene products. | FEMS microbiology letters 20020219 |
| Molecular cloning and characterization of the aklavinone 11-hydroxylase gene of Streptomyces peucetius subsp. caesius ATCC 27952. | Journal of bacteriology 19941101 |
| Isolation and characterization of aclacinomycin A-non-producing Streptomyces galilaeus (ATCC 31615) mutants. | Microbiology (Reading, England) 19940601 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







