200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 162042-44-6
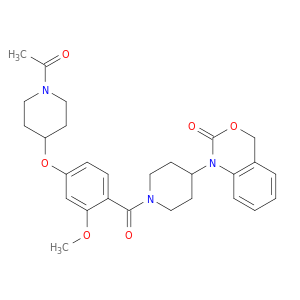
162042-44-6 | 2H-3,1-Benzoxazin-2-one, 1-[1-[4-[(1-acetyl-4-piperidinyl)oxy]-2-methoxybenzoyl]-4-piperidinyl]-1,4-dihydro-
CAS No: 162042-44-6 Catalog No: AG001SOD MDL No:
Product Description
Catalog Number:
AG001SOD
Chemical Name:
2H-3,1-Benzoxazin-2-one, 1-[1-[4-[(1-acetyl-4-piperidinyl)oxy]-2-methoxybenzoyl]-4-piperidinyl]-1,4-dihydro-
CAS Number:
162042-44-6
Molecular Formula:
C28H33N3O6
Molecular Weight:
507.5781
IUPAC Name:
1-[1-[4-(1-acetylpiperidin-4-yl)oxy-2-methoxybenzoyl]piperidin-4-yl]-4H-3,1-benzoxazin-2-one
InChI:
InChI=1S/C28H33N3O6/c1-19(32)29-15-11-22(12-16-29)37-23-7-8-24(26(17-23)35-2)27(33)30-13-9-21(10-14-30)31-25-6-4-3-5-20(25)18-36-28(31)34/h3-8,17,21-22H,9-16,18H2,1-2H3
InChI Key:
WDERJSQJYIJOPD-UHFFFAOYSA-N
SMILES:
COc1cc(ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2c1cccc2)OC1CCN(CC1)C(=O)C
Properties
Complexity:
823
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
507.237g/mol
Formal Charge:
0
Heavy Atom Count:
37
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
507.587g/mol
Monoisotopic Mass:
507.237g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
88.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| Oral oxytocin antagonists. | Journal of medicinal chemistry 20100923 |
| Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist. | British journal of pharmacology 20051101 |
| A Gly/Ala switch contributes to high affinity binding of benzoxazinone-based non-peptide oxytocin receptor antagonists. | FEBS letters 20050117 |
| Identification of potent and selective oxytocin antagonists. Part 1: indole and benzofuran derivatives. | Bioorganic & medicinal chemistry letters 20020520 |
| Identification of potent and selective oxytocin antagonists. Part 2: further investigation of benzofuran derivatives. | Bioorganic & medicinal chemistry letters 20020520 |
| Spontaneous contractions of myometrium from humans, non-human primate and rodents are sensitive to selective oxytocin receptor antagonism in vitro. | BJOG : an international journal of obstetrics and gynaecology 20010901 |
| Development of orally active oxytocin antagonists: studies on 1-(1-[4-[1-(2-methyl-1-oxidopyridin-3-ylmethyl)piperidin-4-yloxy]-2- methoxybenzoyl]piperidin-4-yl)-1,4-dihydrobenz[d][1,3]oxazin-2-one (L-372,662) and related pyridines. | Journal of medicinal chemistry 19980604 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.