200,000+ products from a single source!
sales@angenechem.com
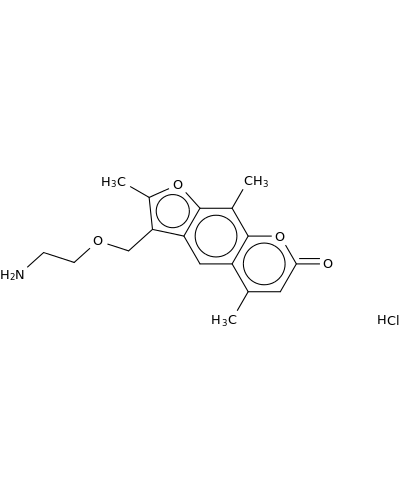
161262-45-9 | 7H-Furo[3,2-g][1]benzopyran-7-one, 3-[(2-aminoethoxy)methyl]-2,5,9-trimethyl-, hydrochloride (1:1)
CAS No: 161262-45-9 Catalog No: AG001SAC MDL No:
Product Description
Catalog Number:
AG001SAC
Chemical Name:
7H-Furo[3,2-g][1]benzopyran-7-one, 3-[(2-aminoethoxy)methyl]-2,5,9-trimethyl-, hydrochloride (1:1)
CAS Number:
161262-45-9
Molecular Formula:
C17H20ClNO4
Molecular Weight:
337.7980
IUPAC Name:
3-(2-aminoethoxymethyl)-2,5,9-trimethylfuro[3,2-g]chromen-7-one;hydrochloride
InChI:
InChI=1S/C17H19NO4.ClH/c1-9-6-15(19)22-16-10(2)17-13(7-12(9)16)14(11(3)21-17)8-20-5-4-18;/h6-7H,4-5,8,18H2,1-3H3;1H
InChI Key:
MHLAMQBABOJZQW-UHFFFAOYSA-N
SMILES:
NCCOCc1c(C)oc2c1cc1c(C)cc(=O)oc1c2C.Cl
UNII:
67B255SI5F
Properties
Complexity:
464
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
2
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
337.108g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
337.8g/mol
Monoisotopic Mass:
337.108g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
74.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Therapeutic efficacy of platelet components treated with amotosalen and ultraviolet A pathogen inactivation method: results of a meta-analysis of randomized controlled trials. | Vox sanguinis 20121101 |
| Pathogen inactivation technology applied to a blood component collected from an asymptomatic carrier of Leishmania infantum: a case report. | Vox sanguinis 20121101 |
| Killed but metabolically active Leishmania infantum as a novel whole-cell vaccine for visceral leishmaniasis. | Clinical and vaccine immunology : CVI 20120401 |
| Bacterial contamination of platelet components: potential solutions to prevent transfusion-related sepsis. | Expert review of hematology 20111001 |
| [Pathogen inactivation in platelet concentrates: the French experience]. | Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine 20110801 |
| A multi-centre study of therapeutic efficacy and safety of platelet components treated with amotosalen and ultraviolet A pathogen inactivation stored for 6 or 7 d prior to transfusion. | British journal of haematology 20110501 |
| [Photochemical inactivation of pathogens in platelets and plasma: five years of clinical use in routine and hemovigilance. Towards a change of paradigm in transfusion safety]. | Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine 20110401 |
| A comparison of methods of pathogen inactivation of FFP. | Vox sanguinis 20110201 |
| In vitro assessment of apheresis and pooled buffy coat platelet components suspended in plasma and SSP+ photochemically treated with amotosalen and UVA for pathogen inactivation (INTERCEPT Blood System™). | Vox sanguinis 20110201 |
| The extent of amotosalen photodegradation during photochemical treatment of platelet components correlates with the level of pathogen inactivation. | Transfusion 20110101 |
| Monitoring photochemical pathogen inactivation treatment using amotosalen and ultraviolet-A light: evaluation of an indicator label. | Vox sanguinis 20101101 |
| An active hemovigilance program characterizing the safety profile of 7483 transfusions with plasma components prepared with amotosalen and UVA photochemical treatment. | Transfusion 20100601 |
| INTERCEPT plasma: comparability with conventional fresh-frozen plasma based on coagulation function--an in vitro analysis. | Vox sanguinis 20100101 |
| Functional characteristics of apheresis-derived platelets treated with ultraviolet light combined with either amotosalen-HCl (S-59) or riboflavin (vitamin B2) for pathogen-reduction. | Vox sanguinis 20090701 |
| Assessment of safety in neonates for transfusion of platelets and plasma prepared with amotosalen photochemical pathogen inactivation treatment by a 1-month intravenous toxicity study in neonatal rats. | Transfusion 20090501 |
| Killed but metabolically active Bacillus anthracis vaccines induce broad and protective immunity against anthrax. | Infection and immunity 20090401 |
| Evaluation of in vitro storage properties of prestorage pooled whole blood-derived platelets suspended in 100 percent plasma and treated with amotosalen and long-wavelength ultraviolet light. | Transfusion 20090401 |
| Quantitative analysis of DNA interstrand cross-links and monoadducts formed in human cells induced by psoralens and UVA irradiation. | Analytical chemistry 20081115 |
| Quantitative and qualitative analysis of proteins in fresh frozen plasma obtained from whole blood donations and prepared with two photochemical treatments. | Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis 20081001 |
| Photochemical inactivation with amotosalen and long-wavelength ultraviolet light of Plasmodium and Babesia in platelet and plasma components. | Transfusion 20080801 |
| Effects of Mirasol PRT treatment on storage lesion development in plasma-stored apheresis-derived platelets compared to untreated and irradiated units. | Transfusion 20080801 |
| An active haemovigilance programme characterizing the safety profile of 7437 platelet transfusions prepared with amotosalen photochemical treatment. | Vox sanguinis 20080501 |
| Photochemical treatment of plasma with amotosalen and UVA light: process validation in three European blood centers. | Transfusion 20080401 |
| Coagulation function in fresh-frozen plasma prepared with two photochemical treatment methods: methylene blue and amotosalen. | Transfusion 20080101 |
| Pathogen inactivation: a new paradigm for preventing transfusion-transmitted infections. | American journal of clinical pathology 20071201 |
| The pathogen reduction treatment of platelets with S-59 HCl (Amotosalen) plus ultraviolet A light: genotoxicity profile and hazard assessment. | Mutation research 20070615 |
| Inactivation of parvovirus B19 in human platelet concentrates by treatment with amotosalen and ultraviolet A illumination. | Transfusion 20070601 |
| Leukoreduced buffy coat-derived platelet concentrates photochemically treated with amotosalen HCl and ultraviolet A light stored up to 7 days: assessment of hemostatic function under flow conditions. | Transfusion 20070401 |
| The efficacy of photochemical treatment with amotosalen HCl and ultraviolet A (INTERCEPT) for inactivation of Trypanosoma cruzi in pooled buffy-coat platelets. | Transfusion 20070301 |
| Quantification of viral inactivation by photochemical treatment with amotosalen and UV A light, using a novel polymerase chain reaction inhibition method with preamplification. | The Journal of infectious diseases 20061215 |
| A randomized, controlled Phase III trial of therapeutic plasma exchange with fresh-frozen plasma (FFP) prepared with amotosalen and ultraviolet A light compared to untreated FFP in thrombotic thrombocytopenic purpura. | Transfusion 20061001 |
| Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. | Transfusion 20060701 |
| Platelets photochemically treated with amotosalen HCl and ultraviolet A light correct prolonged bleeding times in patients with thrombocytopenia. | Transfusion 20060501 |
| Pathogen inactivation of platelets with a photochemical treatment with amotosalen HCl and ultraviolet light: process used in the SPRINT trial. | Transfusion 20060401 |
| Transfusion of 7-day-old amotosalen photochemically treated buffy-coat platelets to patients with thrombocytopenia: a pilot study. | Transfusion 20060301 |
| In vitro evaluation of Haemonetics MCS+ apheresis platelet concentrates treated with photochemical pathogen inactivation following plasma volume reduction using the INTERCEPT Preparation Set. | Vox sanguinis 20060201 |
| The role of photochemical treatment with amotosalen and UV-A light in the prevention of transfusion-transmitted cytomegalovirus infections. | Transfusion medicine reviews 20060101 |
| Platelet dose consistency and its effect on the number of platelet transfusions for support of thrombocytopenia: an analysis of the SPRINT trial of platelets photochemically treated with amotosalen HCl and ultraviolet A light. | Transfusion 20060101 |
| Effect of the psoralen-based photochemical pathogen inactivation on mitochondrial DNA in platelets. | Platelets 20051201 |
| Clinical safety of platelets photochemically treated with amotosalen HCl and ultraviolet A light for pathogen inactivation: the SPRINT trial. | Transfusion 20051201 |
| Amotosalen interactions with platelet and plasma components: absence of neoantigen formation after photochemical treatment. | Transfusion 20051001 |
| Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. | Transfusion 20050901 |
| Leishmania inactivation in human pheresis platelets by a psoralen (amotosalen HCl) and long-wavelength ultraviolet irradiation. | Transfusion 20050901 |
| Polymerase chain reaction inhibition assay documenting the amotosalen-based photochemical pathogen inactivation process of platelet concentrates. | Transfusion 20050901 |
| Fresh frozen plasma prepared with amotosalen HCl (S-59) photochemical pathogen inactivation: transfusion of patients with congenital coagulation factor deficiencies. | Transfusion 20050801 |
| Amotosalen photochemical inactivation of severe acute respiratory syndrome coronavirus in human platelet concentrates. | Transfusion medicine (Oxford, England) 20050801 |
| In vitro photochemical inactivation of cell-associated human T-cell leukemia virus Type I and II in human platelet concentrates and plasma by use of amotosalen. | Transfusion 20050701 |
| Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. | Transfusion 20050401 |
| Protecting the blood supply from emerging pathogens: the role of pathogen inactivation. | Transfusion medicine reviews 20050401 |
| Amotosalen-treated donor T cells have polyclonal antigen-specific long-term function without graft-versus-host disease after allogeneic bone marrow transplantation. | Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 20050301 |
| Stem cell transplantation with S-59 photochemically treated T-cell add-backs to establish allochimerism in murine thalassemia. | Annals of the New York Academy of Sciences 20050101 |
| Recovery and life span of 111indium-radiolabeled platelets treated with pathogen inactivation with amotosalen HCl (S-59) and ultraviolet A light. | Transfusion 20041201 |
| Photochemical treatment of platelet concentrates with amotosalen and long-wavelength ultraviolet light inactivates a broad spectrum of pathogenic bacteria. | Transfusion 20041001 |
| Implementation of the INTERCEPT Blood System for Platelets into routine blood bank manufacturing procedures: evaluation of apheresis platelets. | Vox sanguinis 20040501 |
| In vitro evaluation of pooled buffy coat platelets treated with photochemical pathogen inactivation using amotosalen. | Vox sanguinis 20040401 |
| Functional characteristics of photochemically treated platelets. | Transfusion 20040301 |
| Functional characteristics of buffy-coat PLTs photochemically treated with amotosalen-HCl for pathogen inactivation. | Transfusion 20040301 |
| In vitro evaluation of COM.TEC apheresis platelet concentrates using a preparation set and pathogen inactivation over a storage period of five days. | Journal of clinical apheresis 20040101 |
| Allogeneic T cells treated with amotosalen prevent lethal cytomegalovirus disease without producing graft-versus-host disease following bone marrow transplantation. | Journal of immunology (Baltimore, Md. : 1950) 20031201 |
| Preclinical safety profile of plasma prepared using the INTERCEPT Blood System. | Vox sanguinis 20031001 |
| Safety of the blood supply: role of pathogen reduction. | Blood reviews 20030601 |
| Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. | Blood 20030315 |
| Amotosalen: Allogeneic Cellular Immunotherapies system, INTERCEPT Plasma System, INTERCEPT Platelet System, S 59. | BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy 20030101 |
| Multilineage engraftment with minimal graft-versus-host disease following in utero transplantation of S-59 psoralen/ultraviolet a light-treated, sensitized T cells and adult T cell-depleted bone marrow in fetal mice. | Journal of immunology (Baltimore, Md. : 1950) 20021201 |
| Pharmacokinetic study of FFP photochemically treated with amotosalen (S-59) and UV light compared to FFP in healthy volunteers anticoagulated with warfarin. | Transfusion 20021001 |
| [Blood donation]. | Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine 20020701 |
| Preclinical safety of a nucleic acid-targeted Helinx compound: a clinical perspective. | Seminars in hematology 20011001 |
| Pharmacokinetic and toxicology assessment of INTERCEPT (S-59 and UVA treated) platelets. | Human & experimental toxicology 20011001 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







