200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 16090-33-8
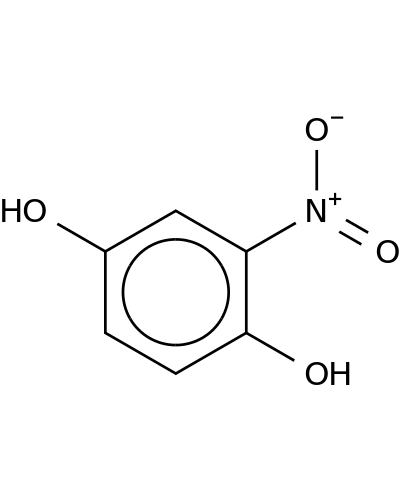
16090-33-8 | 1,4-Benzenediol, 2-nitro-
CAS No: 16090-33-8 Catalog No: AG001VGZ MDL No:MFCD00269642
Product Description
Catalog Number:
AG001VGZ
Chemical Name:
1,4-Benzenediol, 2-nitro-
CAS Number:
16090-33-8
Molecular Formula:
C6H5NO4
Molecular Weight:
155.1082
MDL Number:
MFCD00269642
IUPAC Name:
2-nitrobenzene-1,4-diol
InChI:
InChI=1S/C6H5NO4/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,8-9H
InChI Key:
VIIYYMZOGKODQG-UHFFFAOYSA-N
SMILES:
Oc1ccc(c(c1)[N+](=O)[O-])O
NSC Number:
138350
Properties
Complexity:
155
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
155.022g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
155.109g/mol
Monoisotopic Mass:
155.022g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
86.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.6
Literature
| Title | Journal |
|---|---|
| 2-Nitro-p-phenyl-ene dibenzene-sulfonate. | Acta crystallographica. Section E, Structure reports online 20100201 |
| Genotoxic mechanisms for the carcinogenicity of the environmental pollutants and carcinogens o-anisidine and 2-nitroanisole follow from adducts generated by their metabolite N-(2-methoxyphenyl)-hydroxylamine with deoxyguanosine in DNA. | Interdisciplinary toxicology 20090301 |
| Rat cytochromes P450 oxidize 2-nitrophenol, a human metabolite of carcinogenic 2-nitroanisole. | Neuro endocrinology letters 20090101 |
| 2-Nitro-p-phenyl-ene bis-(toluene-sulfonate). | Acta crystallographica. Section E, Structure reports online 20080901 |
| Oxidation of carcinogenic 2-nitroanisole by rat cytochromes P450 - similarity between human and rat enzymes. | Interdisciplinary toxicology 20080901 |
| Oxidative detoxication of carcinogenic 2-nitroanisole by human, rat and rabbit cytochrome P450. | Neuro endocrinology letters 20061201 |
| Protein engineering of toluene-o-xylene monooxygenase from Pseudomonas stutzeri OX1 for oxidizing nitrobenzene to 3-nitrocatechol, 4-nitrocatechol, and nitrohydroquinone. | Journal of biotechnology 20050126 |
| Direct photolysis of nitroaromatic compounds in aqueous solutions. | Journal of environmental sciences (China) 20050101 |
| Photodegradation of nitroaromatic compounds in aqueous solutions in the UV/ H2O2 process. | Journal of environmental sciences (China) 20050101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.