200,000+ products from a single source!
sales@angenechem.com
Home > Aldehydes > 159091-35-7
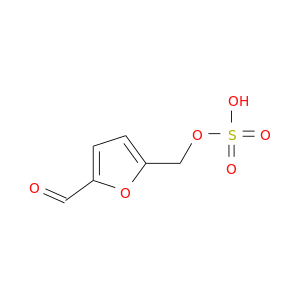
159091-35-7 | 2-Furancarboxaldehyde, 5-[(sulfooxy)methyl]-
CAS No: 159091-35-7 Catalog No: AG001QGC MDL No:
Product Description
Catalog Number:
AG001QGC
Chemical Name:
2-Furancarboxaldehyde, 5-[(sulfooxy)methyl]-
CAS Number:
159091-35-7
Molecular Formula:
C6H6O6S
Molecular Weight:
206.1732
IUPAC Name:
(5-formylfuran-2-yl)methyl hydrogen sulfate
InChI:
InChI=1S/C6H6O6S/c7-3-5-1-2-6(12-5)4-11-13(8,9)10/h1-3H,4H2,(H,8,9,10)
InChI Key:
WVMJEBICTINBRO-UHFFFAOYSA-N
SMILES:
O=Cc1ccc(o1)COS(=O)(=O)O
Properties
Complexity:
265
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
205.989g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
206.168g/mol
Monoisotopic Mass:
205.989g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
102A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.5
Literature
| Title | Journal |
|---|---|
| Mutagenicity of 5-hydroxymethylfurfural in V79 cells expressing human SULT1A1: identification and mass spectrometric quantification of DNA adducts formed. | Chemical research in toxicology 20120716 |
| Toxicity studies with 5-hydroxymethylfurfural and its metabolite 5-sulphooxymethylfurfural in wild-type mice and transgenic mice expressing human sulphotransferases 1A1 and 1A2. | Archives of toxicology 20120501 |
| Study of 5-hydroxymethylfurfural and its metabolite 5-sulfooxymethylfurfural on induction of colonic aberrant crypt foci in wild-type mice and transgenic mice expressing human sulfotransferases 1A1 and 1A2. | Molecular nutrition & food research 20120401 |
| Hydroxymethyl-substituted furans: mutagenicity in Salmonella typhimurium strains engineered for expression of various human and rodent sulphotransferases. | Mutagenesis 20120101 |
| Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20100201 |
| Renal organic anion transporters OAT1 and OAT3 mediate the cellular accumulation of 5-sulfooxymethylfurfural, a reactive, nephrotoxic metabolite of the Maillard product 5-hydroxymethylfurfural. | Biochemical pharmacology 20090815 |
| 5-Hydroxymethylfurfural and 5-sulfooxymethylfurfural increase adenoma and flat ACF number in the intestine of Min/+ mice. | Anticancer research 20090601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







