200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1576-87-0
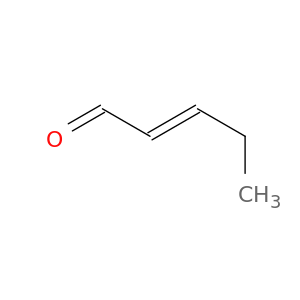
1576-87-0 | 2-Pentenal, (2E)-
CAS No: 1576-87-0 Catalog No: AG001PJR MDL No:MFCD00009615
Product Description
Catalog Number:
AG001PJR
Chemical Name:
2-Pentenal, (2E)-
CAS Number:
1576-87-0
Molecular Formula:
C5H8O
Molecular Weight:
84.1164
MDL Number:
MFCD00009615
IUPAC Name:
(E)-pent-2-enal
InChI:
InChI=1S/C5H8O/c1-2-3-4-5-6/h3-5H,2H2,1H3/b4-3+
InChI Key:
DTCCTIQRPGSLPT-ONEGZZNKSA-N
SMILES:
CC/C=C/C=O
UNII:
7A4R3CQA2T
FEMA Number:
3218
Properties
Complexity:
55
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
84.058g/mol
Formal Charge:
0
Heavy Atom Count:
6
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
84.118g/mol
Monoisotopic Mass:
84.058g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
17.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| NADPH-dependent reductases involved in the detoxification of reactive carbonyls in plants. | The Journal of biological chemistry 20110304 |
| Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. | Journal of medicinal chemistry 20100722 |
| Comparison of tomatillo and tomato volatile compounds in the headspace by selected ion flow tube mass spectrometry (SIFT-MS). | Journal of food science 20100401 |
| Multiple cation channels mediate increases in intracellular calcium induced by the volatile irritant, trans-2-pentenal in rat trigeminal neurons. | Cellular and molecular neurobiology 20100101 |
| cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. | Proceedings of the National Academy of Sciences of the United States of America 20080325 |
| Rate coefficients for the reaction of OH with (E)-2-pentenal, (E)-2-hexenal, and (E)-2-heptenal. | Physical chemistry chemical physics : PCCP 20070614 |
| Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. | Nature 20070201 |
| Volatile constituents from the leaves of Callicarpa japonica Thunb. and their antibacterial activities. | Journal of agricultural and food chemistry 20040225 |
| Antioxidative function and substrate specificity of NAD(P)H-dependent alkenal/one oxidoreductase. A new role for leukotriene B4 12-hydroxydehydrogenase/15-oxoprostaglandin 13-reductase. | The Journal of biological chemistry 20011102 |
| Characterization of the glutathione binding site of aldose reductase. | Chemico-biological interactions 20010130 |
| Structural and kinetic determinants of aldehyde reduction by aldose reductase. | Biochemistry 19990105 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.