200,000+ products from a single source!
sales@angenechem.com
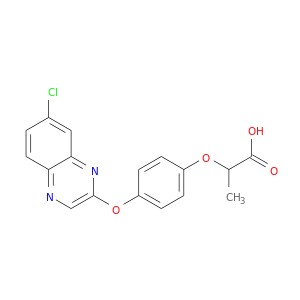
157435-10-4 | Propanoic acid, 2-[4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy]-
CAS No: 157435-10-4 Catalog No: AG001PGE MDL No:
Product Description
Catalog Number:
AG001PGE
Chemical Name:
Propanoic acid, 2-[4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy]-
CAS Number:
157435-10-4
Molecular Formula:
C17H13ClN2O4
Molecular Weight:
344.7491
IUPAC Name:
2-[4-(7-chloroquinoxalin-2-yl)oxyphenoxy]propanoic acid
InChI:
InChI=1S/C17H13ClN2O4/c1-10(17(21)22)23-12-3-5-13(6-4-12)24-16-9-19-14-7-2-11(18)8-15(14)20-16/h2-10H,1H3,(H,21,22)
InChI Key:
NUQZXROIVGBRGR-UHFFFAOYSA-N
SMILES:
CC(C(=O)O)Oc1ccc(cc1)Oc1cnc2c(n1)cc(cc2)Cl
Properties
Complexity:
431
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
344.056g/mol
Formal Charge:
0
Heavy Atom Count:
24
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
344.751g/mol
Monoisotopic Mass:
344.056g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
81.5A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
3.6
Literature
| Title | Journal |
|---|---|
| The role of autophagy in the death of L1210 leukemia cells initiated by the new antitumor agents, XK469 and SH80. | Molecular cancer therapeutics 20070101 |
| Synthesis and biological evaluation of conformationally constrained analogs of the antitumor agents XK469 and SH80. Part 5. | Bioorganic & medicinal chemistry 20060401 |
| Synthetic modification of the 2-oxypropionic acid moiety in 2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}propionic acid (XK469), and consequent antitumor effects. Part 4. | Bioorganic & medicinal chemistry 20050602 |
| Part 3: synthesis and biological evaluation of some analogs of the antitumor agents, 2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}propionic acid, and 2-{4-[(7-bromo-2-quinolinyl)oxy]phenoxy}propionic acid. | Bioorganic & medicinal chemistry 20050215 |
| Preclinical evaluation of 2-[4-(7-chloro-2-quinoxalinyloxy)phenoxy]-propionic acid as a modulator of etoposide in human Waldenstrom's macroglobulinemia xenograft model. | Clinical cancer research : an official journal of the American Association for Cancer Research 20031115 |
| 2-[4-(7-chloro-2-quinoxalinyloxy)phenoxy]-propionic acid (XK469) inhibition of topoisomerase IIbeta is not sufficient for therapeutic response in human Waldenstrom's macroglobulinemia xenograft model. | Molecular cancer therapeutics 20021201 |
| II. Synthesis and biological evaluation of some bioisosteres and congeners of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy)propionic acid (XK469). | Journal of medicinal chemistry 20020704 |
| The investigational new drug XK469 induces G(2)-M cell cycle arrest by p53-dependent and -independent pathways. | Clinical cancer research : an official journal of the American Association for Cancer Research 20011101 |
| Pro-apoptotic interactions between XK469 and the peripheral benzodiazepine receptor. | Cancer letters 20010726 |
| Design, synthesis, and biological evaluation of analogues of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy)propionic acid (XK469). | Journal of medicinal chemistry 20010524 |
Related Products
© 2019 Angene International Limited. All rights Reserved.