200,000+ products from a single source!
sales@angenechem.com
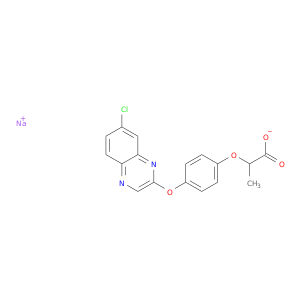
157434-99-6 | Propanoic acid, 2-[4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy]-, sodium salt (1:1)
CAS No: 157434-99-6 Catalog No: AG001PGF MDL No:
Product Description
Catalog Number:
AG001PGF
Chemical Name:
Propanoic acid, 2-[4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy]-, sodium salt (1:1)
CAS Number:
157434-99-6
Molecular Formula:
C17H12ClN2NaO4
Molecular Weight:
366.7310
IUPAC Name:
sodium;2-[4-(7-chloroquinoxalin-2-yl)oxyphenoxy]propanoate
InChI:
InChI=1S/C17H13ClN2O4.Na/c1-10(17(21)22)23-12-3-5-13(6-4-12)24-16-9-19-14-7-2-11(18)8-15(14)20-16;/h2-10H,1H3,(H,21,22);/q;+1/p-1
InChI Key:
OJENKXNXJPNEPU-UHFFFAOYSA-M
SMILES:
CC(C(=O)[O-])Oc1ccc(cc1)Oc1cnc2c(n1)cc(cc2)Cl.[Na+]
Properties
Complexity:
437
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
2
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
366.038g/mol
Formal Charge:
0
Heavy Atom Count:
25
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
366.733g/mol
Monoisotopic Mass:
366.038g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
84.4A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| R(+)XK469 inhibits hydroxylation of S-warfarin by CYP2C9. | European journal of cancer (Oxford, England : 1990) 20090701 |
| The chemotherapeutic agents XK469 (2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}propionic acid) and SH80 (2-{4-[(7-bromo-2-quinolinyl)oxy]phenoxy}propionic acid) inhibit cytokinesis and promote polyploidy and induce senescence. | The Journal of pharmacology and experimental therapeutics 20090301 |
| [Mechanisms of G2/M cycle arrest induced by topo IIalpha and II beta inhibitors in H460 cells]. | Nan fang yi ke da xue xue bao = Journal of Southern Medical University 20081201 |
| A phase I and pharmacokinetic study of XK469R (NSC 698215), a quinoxaline phenoxypropionic acid derivative, in patients with refractory acute leukemia. | Investigational new drugs 20080801 |
| A phase I and pharmacokinetic study of the quinoxaline antitumour Agent R(+)XK469 in patients with advanced solid tumours. | European journal of cancer (Oxford, England : 1990) 20080801 |
| Ethonafide-induced cytotoxicity is mediated by topoisomerase II inhibition in prostate cancer cells. | The Journal of pharmacology and experimental therapeutics 20070601 |
| A phase 1 trial of XK469: toxicity profile of a selective topoisomerase IIbeta inhibitor. | Investigational new drugs 20070401 |
| The role of autophagy in the death of L1210 leukemia cells initiated by the new antitumor agents, XK469 and SH80. | Molecular cancer therapeutics 20070101 |
| Synthesis and biological evaluation of conformationally constrained analogs of the antitumor agents XK469 and SH80. Part 5. | Bioorganic & medicinal chemistry 20060401 |
| Solubilization of two structurally related anticancer drugs: XK-469 and PPA. | Journal of pharmaceutical sciences 20060101 |
| Metabolic profile of XK469 (2(R)-[4-(7-chloro-2-quinoxalinyl)oxyphenoxy]-propionic acid; NSC698215) in patients and in vitro: low potential for active or toxic metabolites or for drug-drug interactions. | Cancer chemotherapy and pharmacology 20051001 |
| Synthetic modification of the 2-oxypropionic acid moiety in 2-{4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy}propionic acid (XK469), and consequent antitumor effects. Part 4. | Bioorganic & medicinal chemistry 20050602 |
| [Effect of XK469 and adriamycin on the growth of H460 cells in vitro and its mechanism]. | Di 1 jun yi da xue xue bao = Academic journal of the first medical college of PLA 20040701 |
| XK469, a topo IIbeta inhibitor, induces apoptosis in Waldenstrom's macroglobulinemia through multiple pathways. | International journal of oncology 20031201 |
| Preclinical evaluation of 2-[4-(7-chloro-2-quinoxalinyloxy)phenoxy]-propionic acid as a modulator of etoposide in human Waldenstrom's macroglobulinemia xenograft model. | Clinical cancer research : an official journal of the American Association for Cancer Research 20031115 |
| DNA sequence specificity for topoisomerase II poisoning by the quinoxaline anticancer drugs XK469 and CQS. | Molecular pharmacology 20030601 |
| 2-[4-(7-chloro-2-quinoxalinyloxy)phenoxy]-propionic acid (XK469) inhibition of topoisomerase IIbeta is not sufficient for therapeutic response in human Waldenstrom's macroglobulinemia xenograft model. | Molecular cancer therapeutics 20021201 |
| 2-[4-(7-chloro-2-quinoxalinyloxyphenoxy]-propionic acid (XK469), an inhibitor of topoisomerase (Topo) IIbeta, up-regulates Topo IIalpha and enhances Topo IIalpha-mediated cytotoxicity. | Molecular cancer therapeutics 20021201 |
| Induction of apoptosis by the new anticancer drug XK469 in human ovarian cancer cell lines. | Oncogene 20020704 |
| II. Synthesis and biological evaluation of some bioisosteres and congeners of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy)propionic acid (XK469). | Journal of medicinal chemistry 20020704 |
| Preclinical efficacy evaluations of XK-469: dose schedule, route and cross-resistance behavior in tumor bearing mice. | Investigational new drugs 20020201 |
| Mitotic arrest induced by XK469, a novel antitumor agent, is correlated with the inhibition of cyclin B1 ubiquitination. | International journal of cancer 20020101 |
| Cellular drug action profile paradigm applied to XK469. | Journal of experimental therapeutics & oncology 20020101 |
| The investigational new drug XK469 induces G(2)-M cell cycle arrest by p53-dependent and -independent pathways. | Clinical cancer research : an official journal of the American Association for Cancer Research 20011101 |
| Pro-apoptotic interactions between XK469 and the peripheral benzodiazepine receptor. | Cancer letters 20010726 |
| Design, synthesis, and biological evaluation of analogues of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy)propionic acid (XK469). | Journal of medicinal chemistry 20010524 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.