200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1568-65-6
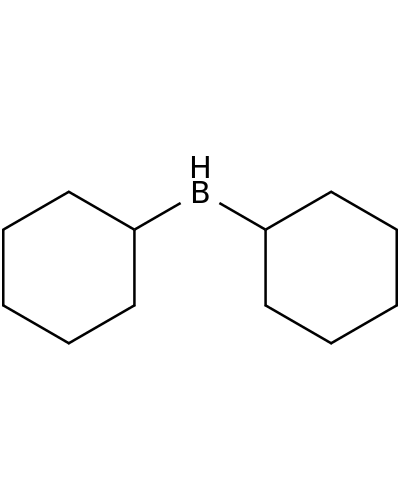
1568-65-6 | Borane, dicyclohexyl-
CAS No: 1568-65-6 Catalog No: AG001P0Z MDL No:
Product Description
Catalog Number:
AG001P0Z
Chemical Name:
Borane, dicyclohexyl-
CAS Number:
1568-65-6
Molecular Formula:
C12H23B
Molecular Weight:
178.1220
InChI:
InChI=1S/C12H22B/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h11-12H,1-10H2
InChI Key:
LUHUASWMJCSDTG-UHFFFAOYSA-N
SMILES:
C1CCC(CC1)BC1CCCCC1
Properties
Complexity:
116
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
177.181g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
177.118g/mol
Monoisotopic Mass:
177.181g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Structure and bonding nature of the strained Lewis acid 3-methyl-1-boraadamantane: a case study employing a new data-analysis procedure in gas electron diffraction. | Chemistry (Weinheim an der Bergstrasse, Germany) 20120820 |
| Catalytic enantioselective synthesis of secondary allylic alcohols from terminal alkynes and aldehydes via 1-alkenylboron reagents. | Organic letters 20101119 |
| Applications of 1-alkenyl-1,1-heterobimetallics in the stereoselective synthesis of cyclopropylboronate esters, trisubstituted cyclopropanols and 2,3-disubstituted cyclobutanones. | Journal of the American Chemical Society 20090513 |
| Highly stereoselective synthesis of cis-alkenyl pinacolboronates and potassium cis-alkenyltrifluoroborates via a hydroboration/protodeboronation approach. | The Journal of organic chemistry 20080905 |
| Generation and tandem reactions of 1-alkenyl-1,1-heterobimetallics: practical and versatile reagents for organic synthesis. | Journal of the American Chemical Society 20080319 |
| Catalytic asymmetric generation of (Z)-disubstituted allylic alcohols. | Journal of the American Chemical Society 20071226 |
| Direct, stereospecific generation of (Z)-disubstituted allylic alcohols. | Journal of the American Chemical Society 20060802 |
Related Products
© 2019 Angene International Limited. All rights Reserved.