200,000+ products from a single source!
sales@angenechem.com
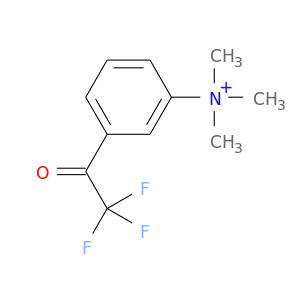
156781-80-5 | Benzenaminium, N,N,N-trimethyl-3-(2,2,2-trifluoroacetyl)-
CAS No: 156781-80-5 Catalog No: AG001OZT MDL No:
Product Description
Catalog Number:
AG001OZT
Chemical Name:
Benzenaminium, N,N,N-trimethyl-3-(2,2,2-trifluoroacetyl)-
CAS Number:
156781-80-5
Molecular Formula:
C11H13F3NO+
Molecular Weight:
232.2222
IUPAC Name:
trimethyl-[3-(2,2,2-trifluoroacetyl)phenyl]azanium
InChI:
InChI=1S/C11H13F3NO/c1-15(2,3)9-6-4-5-8(7-9)10(16)11(12,13)14/h4-7H,1-3H3/q+1
InChI Key:
JIBZSTPMDKSJOX-UHFFFAOYSA-N
SMILES:
O=C(C(F)(F)F)c1cccc(c1)[N+](C)(C)C
Properties
Complexity:
267
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
232.095g/mol
Formal Charge:
1
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
232.226g/mol
Monoisotopic Mass:
232.095g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
17.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.1
Literature
| Title | Journal |
|---|---|
| The significance of low substrate concentration measurements for mechanistic interpretation in cholinesterases. | Chemico-biological interactions 20130325 |
| ENZO: a web tool for derivation and evaluation of kinetic models of enzyme catalyzed reactions. | PloS one 20110101 |
| Acetylcholinesterase: mechanisms of covalent inhibition of H447I mutant determined by computational analyses. | Chemico-biological interactions 20080925 |
| Exploiting protein fluctuations at the active-site gorge of human cholinesterases: further optimization of the design strategy to develop extremely potent inhibitors. | Journal of medicinal chemistry 20080612 |
| qPIPSA: relating enzymatic kinetic parameters and interaction fields. | BMC bioinformatics 20070101 |
| Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding. | The Journal of biological chemistry 20060929 |
| Is aromaticity essential for trapping the catalytic histidine 447 in human acetylcholinesterase? | Biochemistry 20040323 |
| Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate. | Biochemistry 20030513 |
| Studying enzyme binding specificity in acetylcholinesterase using a combined molecular dynamics and multiple docking approach. | Journal of the American Chemical Society 20020717 |
| Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. | The Journal of biological chemistry 20010629 |
| Does 'butyrylization' of acetylcholinesterase through substitution of the six divergent aromatic amino acids in the active center gorge generate an enzyme mimic of butyrylcholinesterase? | Biochemistry 20010626 |
| Short, strong hydrogen bonds at the active site of human acetylcholinesterase: proton NMR studies. | Biochemistry 20010515 |
Related Products
© 2019 Angene International Limited. All rights Reserved.