200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1553-56-6
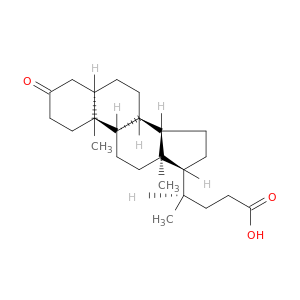
1553-56-6 | Cholan-24-oic acid, 3-oxo-, (5β)-
CAS No: 1553-56-6 Catalog No: AG001NTC MDL No:MFCD00046265
Product Description
Catalog Number:
AG001NTC
Chemical Name:
Cholan-24-oic acid, 3-oxo-, (5β)-
CAS Number:
1553-56-6
Molecular Formula:
C24H38O3
Molecular Weight:
374.5567
MDL Number:
MFCD00046265
IUPAC Name:
(4R)-4-[(5R,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-3-oxo-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pentanoic acid
InChI:
InChI=1S/C24H38O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-16,18-21H,4-14H2,1-3H3,(H,26,27)/t15-,16-,18+,19-,20+,21+,23+,24-/m1/s1
InChI Key:
KIQFUORWRVZTHT-OPTMKGCMSA-N
SMILES:
OC(=O)CC[C@H]([C@H]1CC[C@@H]2[C@]1(C)CC[C@H]1[C@H]2CC[C@H]2[C@]1(C)CCC(=O)C2)C
UNII:
96JBM35FXF
Properties
Complexity:
613
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
8
Defined Bond Stereocenter Count:
0
Exact Mass:
374.282g/mol
Formal Charge:
0
Heavy Atom Count:
27
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
374.565g/mol
Monoisotopic Mass:
374.282g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
54.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5.8
Literature
| Title | Journal |
|---|---|
| The evolution of farnesoid X, vitamin D, and pregnane X receptors: insights from the green-spotted pufferfish (Tetraodon nigriviridis) and other non-mammalian species. | BMC biochemistry 20110101 |
| Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. | Journal of medicinal chemistry 20080327 |
| Biotransformation of lithocholic acid by rat hepatic microsomes: metabolite analysis by liquid chromatography/mass spectrometry. | Drug metabolism and disposition: the biological fate of chemicals 20080201 |
| Rapid and drastic induction of CYP3A4 mRNA expression via vitamin D receptor in human intestinal LS180 cells. | Drug metabolism and pharmacokinetics 20071001 |
| Characterization of an oligomeric carbonyl reductase of dog liver: its identity with peroxisomal tetrameric carbonyl reductase. | Biological & pharmaceutical bulletin 20070901 |
| In silico prediction of pregnane X receptor activators by machine learning approaches. | Molecular pharmacology 20070101 |
| Vitamin D receptor: ligand recognition and allosteric network. | Journal of medicinal chemistry 20060223 |
| Evolution of the pregnane x receptor: adaptation to cross-species differences in biliary bile salts. | Molecular endocrinology (Baltimore, Md.) 20050701 |
| Novel pathways of bile acid metabolism involving CYP3A4. | Biochimica et biophysica acta 20050221 |
| Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. | Journal of lipid research 20050101 |
| Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors). | Nuclear receptor 20050101 |
| Specificity of human alcohol dehydrogenase 1C*2 (gamma2gamma2) for steroids and simulation of the uncompetitive inhibition of ethanol metabolism. | Chemico-biological interactions 20030201 |
| Vitamin D receptor as an intestinal bile acid sensor. | Science (New York, N.Y.) 20020517 |
| The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. | Proceedings of the National Academy of Sciences of the United States of America 20010313 |
| St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. | Proceedings of the National Academy of Sciences of the United States of America 20000620 |
Related Products
© 2019 Angene International Limited. All rights Reserved.