200,000+ products from a single source!
sales@angenechem.com
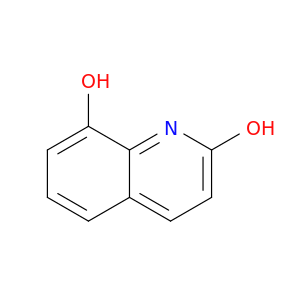
15450-76-7 | 2,8-Quinolinediol
CAS No: 15450-76-7 Catalog No: AG00332D MDL No:MFCD00216696
Product Description
Catalog Number:
AG00332D
Chemical Name:
2,8-Quinolinediol
CAS Number:
15450-76-7
Molecular Formula:
C9H7NO2
Molecular Weight:
161.1574
MDL Number:
MFCD00216696
IUPAC Name:
8-hydroxy-1H-quinolin-2-one
InChI:
InChI=1S/C9H7NO2/c11-7-3-1-2-6-4-5-8(12)10-9(6)7/h1-5,11H,(H,10,12)
InChI Key:
ZXZKYYHTWHJHFT-UHFFFAOYSA-N
SMILES:
Oc1ccc2c(n1)c(O)ccc2
UNII:
466PKO4UBM
NSC Number:
108383
Properties
Complexity:
225
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
161.048g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
161.16g/mol
Monoisotopic Mass:
161.048g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
49.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| Quinoline biodegradation and its nitrogen transformation pathway by a Pseudomonas sp. strain. | Biodegradation 20100601 |
| Interaction of 8-hydroxyquinoline with soil environment mediates its ecological function. | PloS one 20100101 |
| Aerobic biodegradation characteristics and metabolic products of quinoline by a Pseudomonas strain. | Bioresource technology 20091101 |
| [Isolation, identification, and biodegradation characteristics of a quinoline-degrading bacterium]. | Huan jing ke xue= Huanjing kexue 20081201 |
| A new Hg(2+) -selective fluorescent sensor based on a 1,3-alternate thiacalix[4]arene anchored with four 8-quinolinoloxy groups. | Inorganic chemistry 20070806 |
| Configuration-specific synthesis of the facial and meridional isomers of tris(8-hydroxyquinolinate)aluminum (Alq3). | Inorganic chemistry 20060724 |
| Antioxidant effects of quinoline alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. | Biological & pharmaceutical bulletin 20060401 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| 2-Oxoquinoline 8-monooxygenase oxygenase component: active site modulation by Rieske-[2Fe-2S] center oxidation/reduction. | Structure (London, England : 1993) 20050501 |
| Antimicrosporidial activity of (fluoro)quinolones in vitro and in vivo. | Folia parasitologica 20050501 |
| Conversion of tris(8-quinolinolato-N1, O8) aluminum to 8-hydroxyquinoline and activity in bacterial reverse mutation assays. | Mutation research 20050404 |
Related Products
© 2019 Angene International Limited. All rights Reserved.