200,000+ products from a single source!
sales@angenechem.com
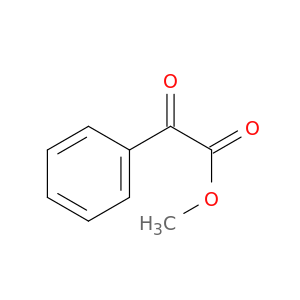
15206-55-0 | Methyl 2-oxo-2-phenylacetate
CAS No: 15206-55-0 Catalog No: AG003RZG MDL No:MFCD00008443
Product Description
Catalog Number:
AG003RZG
Chemical Name:
Methyl 2-oxo-2-phenylacetate
CAS Number:
15206-55-0
Molecular Formula:
C9H8O3
Molecular Weight:
164.1580
MDL Number:
MFCD00008443
IUPAC Name:
methyl 2-oxo-2-phenylacetate
InChI:
InChI=1S/C9H8O3/c1-12-9(11)8(10)7-5-3-2-4-6-7/h2-6H,1H3
InChI Key:
YLHXLHGIAMFFBU-UHFFFAOYSA-N
SMILES:
COC(=O)C(=O)c1ccccc1
EC Number:
239-263-3
UNII:
23F2045STG
Properties
Complexity:
180
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
164.047g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
164.16g/mol
Monoisotopic Mass:
164.047g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
43.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| The first case of competitive heterogeneously catalyzed enantioselective hydrogenation of ketones. | Chemical communications (Cambridge, England) 20110207 |
| Integration of newly isolated biocatalyst and resin-based in situ product removal technique for the asymmetric synthesis of (R)-methyl mandelate. | Bioprocess and biosystems engineering 20100901 |
| Mechanistic insights on the magnesium(II) ion-activated reduction of methyl benzoylformate with chelated NADH peptide beta-lactam models. | The Journal of organic chemistry 20090904 |
| [Overview of 40 years' chemical study]. | Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 20090101 |
| Exploiting nanospace for asymmetric catalysis: confinement of immobilized, single-site chiral catalysts enhances enantioselectivity. | Accounts of chemical research 20080601 |
| N-methyl-(R)-3-(tert-butyl)-sulfinyl-1,4-dihydropyridine: a novel NADH model compound. | Molecules (Basel, Switzerland) 20070312 |
| Unusual temperature dependence of enantioselectivity in asymmetric reductions by chiral NADH models. | Organic letters 20060511 |
| Amine degradation by 4,5-epoxy-2-decenal in model systems. | Journal of agricultural and food chemistry 20060322 |
| Synthesis, properties, and oxidizing ability of areno-annulated 1,3-dimethyl-10-phenylcyclohepta[4,5]pyrrolo[2,3-d]pyrimidine- 2,4(1,3H)-dionylium ions. | The Journal of organic chemistry 20060106 |
| Ru-Catalyzed asymmetric hydrogenation of alpha-ketoesters with CeCl3.7H2O as additive. | Organic letters 20051124 |
| Stereoselective generation of vicinal stereogenic quaternary centers by photocycloaddition of 5-methoxy oxazoles to alpha-keto esters: synthesis of erythro beta-hydroxy dimethyl aspartates. | Organic & biomolecular chemistry 20040421 |
| Constraining asymmetric organometallic catalysts within mesoporous supports boosts their enantioselectivity. | Journal of the American Chemical Society 20031210 |
| Atropoisomeric quinolinium salt promoting the access to both enantiomeric forms of methyl mandelate: a versatile NADH mimic. | Chemical communications (Cambridge, England) 20021007 |
| The [2+2]-photocycloaddition of aromatic aldehydes and ketones to 3,4-dihydro-2-pyridones: regioselectivity, diastereoselectivity, and reductive ring opening of the product oxetanes. | Chemistry (Weinheim an der Bergstrasse, Germany) 20011015 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.