200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1506-47-4
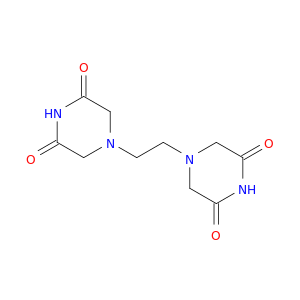
1506-47-4 | 2,6-Piperazinedione, 4,4'-(1,2-ethanediyl)bis-
CAS No: 1506-47-4 Catalog No: AG001MR6 MDL No:MFCD00446913
Product Description
Catalog Number:
AG001MR6
Chemical Name:
2,6-Piperazinedione, 4,4'-(1,2-ethanediyl)bis-
CAS Number:
1506-47-4
Molecular Formula:
C10H14N4O4
Molecular Weight:
254.2426
MDL Number:
MFCD00446913
IUPAC Name:
4-[2-(3,5-dioxopiperazin-1-yl)ethyl]piperazine-2,6-dione
InChI:
InChI=1S/C10H14N4O4/c15-7-3-13(4-8(16)11-7)1-2-14-5-9(17)12-10(18)6-14/h1-6H2,(H,11,15,16)(H,12,17,18)
InChI Key:
GBLIGNUYGOFIKS-UHFFFAOYSA-N
SMILES:
O=C1CN(CCN2CC(=O)NC(=O)C2)CC(=O)N1
UNII:
QML51S42CD
NSC Number:
129942
Properties
Complexity:
339
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
254.102g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
254.246g/mol
Monoisotopic Mass:
254.102g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
98.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.8
Literature
| Title | Journal |
|---|---|
| Antimetastatic activities and mechanisms of bisdioxopiperazine compounds. | Anti-cancer agents in medicinal chemistry 20100901 |
| A mouse model for studying the interaction of bisdioxopiperazines with topoisomerase IIalpha in vivo. | Molecular pharmacology 20071001 |
| Separation of bisdioxopiperazine- and vanadate resistance in topoisomerase II. | Biochemical and biophysical research communications 20050902 |
| Probing the role of linker substituents in bisdioxopiperazine analogs for activity against wild-type and mutant human topoisomerase II alpha. | Molecular pharmacology 20030501 |
| Evidence from studies with intact mammalian cells that merbarone and bis(dioxopiperazine)s are topoisomerase II poisons. | Drug and chemical toxicology 20030201 |
| The catalytic DNA topoisomerase II inhibitor ICRF-193 and all-trans retinoic acid cooperatively induce granulocytic differentiation of acute promyelocytic leukemia cells: candidate drugs for chemo-differentiation therapy against acute promyelocytic leukemia. | Experimental hematology 20021101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.