200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 1504-16-1
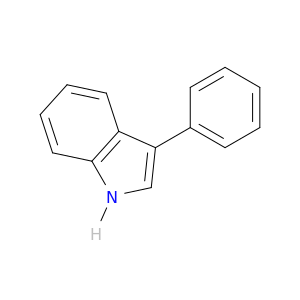
1504-16-1 | 1H-Indole, 3-phenyl-
CAS No: 1504-16-1 Catalog No: AG001ML3 MDL No:
Product Description
Catalog Number:
AG001ML3
Chemical Name:
1H-Indole, 3-phenyl-
CAS Number:
1504-16-1
Molecular Formula:
C14H11N
Molecular Weight:
193.2438
IUPAC Name:
3-phenyl-1H-indole
InChI:
InChI=1S/C14H11N/c1-2-6-11(7-3-1)13-10-15-14-9-5-4-8-12(13)14/h1-10,15H
InChI Key:
XZNGTBLWFCRXKR-UHFFFAOYSA-N
SMILES:
c1ccc(cc1)c1c[nH]c2c1cccc2
UNII:
7676CPK41G
NSC Number:
76690
Properties
Complexity:
207
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
193.089g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
193.249g/mol
Monoisotopic Mass:
193.089g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
15.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.9
Literature
| Title | Journal |
|---|---|
| Structure of the indole-benzene dimer revisited. | The journal of physical chemistry. A 20110901 |
| Accurately characterizing the pi-pi interaction energies of indole-benzene complexes. | The journal of physical chemistry. A 20100318 |
| Substrate specificity and inhibition of brassinin hydrolases, detoxifying enzymes from the plant pathogens Leptosphaeria maculans and Alternaria brassicicola. | The FEBS journal 20091201 |
| The synthesis of 2- and 3-aryl indoles and 1,3,4,5-tetrahydropyrano[4,3-b]indoles and their antibacterial and antifungal activity. | Bioorganic & medicinal chemistry letters 20090901 |
| Synthetic inhibitors of the fungal detoxifying enzyme brassinin oxidase based on the phytoalexin camalexin scaffold. | Journal of agricultural and food chemistry 20090325 |
| Cloning, purification and characterisation of brassinin glucosyltransferase, a phytoalexin-detoxifying enzyme from the plant pathogen Sclerotinia sclerotiorum. | Fungal genetics and biology : FG & B 20090201 |
| Methyl 3-(4-bromo-phen-yl)-2-(1H-indol-3-ylmeth-yl)-5-[1-(4-methoxy-phen-yl)-4-oxo-2-phenyl-azetidin-2-yl]-4-nitro-pyrrolidine-2-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20080601 |
| Design, synthesis, and evaluation of potential inhibitors of brassinin glucosyltransferase, a phytoalexin detoxifying enzyme from Sclerotinia sclerotiorum. | Bioorganic & medicinal chemistry 20070901 |
| Camalexin induces detoxification of the phytoalexin brassinin in the plant pathogen Leptosphaeria maculans. | Phytochemistry 20051101 |
| Protein engineering of toluene ortho-monooxygenase of Burkholderia cepacia G4 for regiospecific hydroxylation of indole to form various indigoid compounds. | Applied microbiology and biotechnology 20050101 |
| Synthesis and antitumor activity of substituted 3-(5-imidazo[2,1-b]thiazolylmethylene)-2-indolinones. | Anti-cancer drug design 20010101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.