200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 14919-49-4
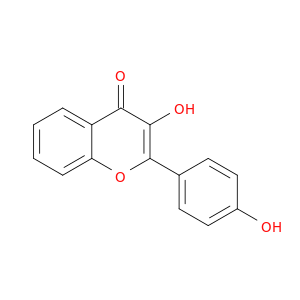
14919-49-4 | 4H-1-Benzopyran-4-one, 3-hydroxy-2-(4-hydroxyphenyl)-
CAS No: 14919-49-4 Catalog No: AG001LG0 MDL No:MFCD00017690
Product Description
Catalog Number:
AG001LG0
Chemical Name:
4H-1-Benzopyran-4-one, 3-hydroxy-2-(4-hydroxyphenyl)-
CAS Number:
14919-49-4
Molecular Formula:
C15H10O4
Molecular Weight:
254.2375
MDL Number:
MFCD00017690
IUPAC Name:
3-hydroxy-2-(4-hydroxyphenyl)chromen-4-one
InChI:
InChI=1S/C15H10O4/c16-10-7-5-9(6-8-10)15-14(18)13(17)11-3-1-2-4-12(11)19-15/h1-8,16,18H
InChI Key:
GPGOCTLAUAHUQO-UHFFFAOYSA-N
SMILES:
Oc1ccc(cc1)c1oc2ccccc2c(=O)c1O
Properties
Complexity:
393
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
254.058g/mol
Formal Charge:
0
Heavy Atom Count:
19
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
254.241g/mol
Monoisotopic Mass:
254.058g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
66.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| Design, synthesis and biological activity of flavonoid derivatives as selective agonists for neuromedin U 2 receptor. | Bioorganic & medicinal chemistry 20141101 |
| A novel relationship between the radical-scavenging activity of flavonoids and enthalpy of formation revealed with Hartree-Fock computations and thermochemical deduction. | Redox report : communications in free radical research 20120101 |
| 3,4-Dihydroxyflavone acts as an antioxidant and antiapoptotic agent to support bovine embryo development in vitro. | The Journal of reproduction and development 20110201 |
| Use of glucuronidation fingerprinting to describe and predict mono- and dihydroxyflavone metabolism by recombinant UGT isoforms and human intestinal and liver microsomes. | Molecular pharmaceutics 20100607 |
| Understanding the cardioprotective effects of flavonols: discovery of relaxant flavonols without antioxidant activity. | Journal of medicinal chemistry 20080327 |
| The anti-apoptotic and anti-oxidant effect of eriodictyol on UV-induced apoptosis in keratinocytes. | Biological & pharmaceutical bulletin 20070101 |
| Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. | The Journal of biological chemistry 20050909 |
Related Products
© 2019 Angene International Limited. All rights Reserved.