200,000+ products from a single source!
sales@angenechem.com
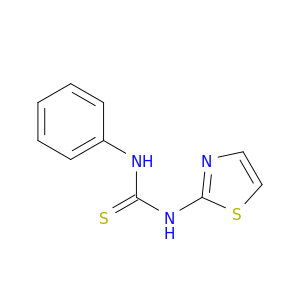
14901-16-7 | Thiourea,N-phenyl-N'-2-thiazolyl-
CAS No: 14901-16-7 Catalog No: AG007JBB MDL No:MFCD00005321
Product Description
Catalog Number:
AG007JBB
Chemical Name:
Thiourea,N-phenyl-N'-2-thiazolyl-
CAS Number:
14901-16-7
Molecular Formula:
C10H9N3S2
Molecular Weight:
235.3286
MDL Number:
MFCD00005321
IUPAC Name:
1-phenyl-3-(1,3-thiazol-2-yl)thiourea
InChI:
InChI=1S/C10H9N3S2/c14-9(13-10-11-6-7-15-10)12-8-4-2-1-3-5-8/h1-7H,(H2,11,12,13,14)
InChI Key:
GCZZOZBWAZHCAN-UHFFFAOYSA-N
SMILES:
S=C(Nc1nccs1)Nc1ccccc1
EC Number:
238-970-4
NSC Number:
139257
Properties
Complexity:
217
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
235.024g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
235.323g/mol
Monoisotopic Mass:
235.024g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
97.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.4
Literature
| Title | Journal |
|---|---|
| Endotoxin is not essential for the development of cockroach induced allergic airway inflammation. | Yonsei medical journal 20120501 |
| Cognitive-enhancing effect of quercetin in a rat model of Parkinson's disease induced by 6-hydroxydopamine. | Evidence-based complementary and alternative medicine : eCAM 20120101 |
| Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. | Nature chemical biology 20091001 |
| Ytterbium-selective polymeric membrane electrode based on substituted urea and thiourea as a suitable carrier. | Analytica chimica acta 20070806 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Structure-based design, parallel synthesis, structure-activity relationship, and molecular modeling studies of thiocarbamates, new potent non-nucleoside HIV-1 reverse transcriptase inhibitor isosteres of phenethylthiazolylthiourea derivatives. | Journal of medicinal chemistry 20050602 |
| Phenyl thiazolyl urea and carbamate derivatives as new inhibitors of bacterial cell-wall biosynthesis. | Bioorganic & medicinal chemistry letters 20040105 |
| Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. | The Journal of biological chemistry 20020419 |
| Effects of a dopamine beta-hydroxylase inhibitor on amphetamine-induced hyperactivity in rats. | The Journal of pharmacy and pharmacology 19750801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.