200,000+ products from a single source!
sales@angenechem.com
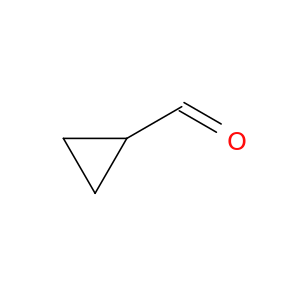
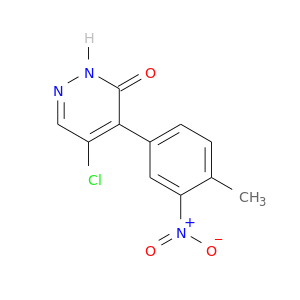
1489-69-6 | Cyclopropanecarboxaldehyde
CAS No: 1489-69-6 Catalog No: AG001LAD MDL No:MFCD00012261
Product Description
Catalog Number:
AG001LAD
Chemical Name:
Cyclopropanecarboxaldehyde
CAS Number:
1489-69-6
Molecular Formula:
C4H6O
Molecular Weight:
70.0898
MDL Number:
MFCD00012261
IUPAC Name:
cyclopropanecarbaldehyde
InChI:
InChI=1S/C4H6O/c5-3-4-1-2-4/h3-4H,1-2H2
InChI Key:
JMYVMOUINOAAPA-UHFFFAOYSA-N
SMILES:
O=CC1CC1
Properties
Complexity:
45.6
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
70.042g/mol
Formal Charge:
0
Heavy Atom Count:
5
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
70.091g/mol
Monoisotopic Mass:
70.042g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
17.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.2
Literature
| Title | Journal |
|---|---|
| Cyclopropylhydroxycarbene. | Journal of the American Chemical Society 20110831 |
| Steric effects on the enantiodiscrimination of diproline chiral stationary phases in the resolution of racemic compounds. | Journal of chromatography. A 20110812 |
| Stereoselective routes to aryl substituted γ-butyrolactones and their application towards the synthesis of highly oxidised furanocembranoids. | Organic & biomolecular chemistry 20110507 |
| Cyclopropane-aldehyde annulations at quaternary donor sites: stereoselective access to highly substituted tetrahydrofurans. | Organic letters 20110415 |
| Chemo-, regio-, and diastereoselectivity preferences in the reaction of a sulfur ylide with a dienal and an enone. | Organic & biomolecular chemistry 20110307 |
| Complexity-building annulations of strained cycloalkanes and C═O π bonds. | The Journal of organic chemistry 20101001 |
| Synthesis and antiproliferative activity of indolizine derivatives incorporating a cyclopropylcarbonyl group against Hep-G2 cancer cell line. | European journal of medicinal chemistry 20100701 |
| N-heterocyclic carbene-catalyzed domino ring-opening/redox amidation/cyclization reactions of formylcyclopropane 1,1-diesters: direct construction of a 6-5-6 tricyclic hydropyrido[1,2-a]indole skeleton. | The Journal of organic chemistry 20090605 |
| Reactions of monoaryl-substituted methylenecyclobutanes and methylenecyclopropanes with 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt), and 1-hydroxysuccinimide (HOSu). | The Journal of organic chemistry 20090320 |
| Action of N-acylated ambroxol derivatives on secretion of chloride ions in human airway epithelia. | Biochemical and biophysical research communications 20090313 |
| Measuring picosecond isomerization kinetics via broadband microwave spectroscopy. | Science (New York, N.Y.) 20080516 |
| One-pot organocatalytic domino Michael/alpha-alkylation reactions: direct catalytic enantioselective cyclopropanation and cyclopentanation reactions. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Cyclopropylmethyl/cyclobutyl rearrangements on surfaces: evidence for transient cation formation near O-covered Mo(110). | Journal of the American Chemical Society 20070418 |
| Cyclopropanation of enantiopure metal alkenyl carbenes with 2-methoxyfuran: a practical route to carboxycyclopropylglycine precursors. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| Enzyme inhibition potency enhancement by active site metal chelating and hydrogen bonding induced conformation-restricted cyclopropanecarbonyl derivatives. | Bioorganic & medicinal chemistry letters 20061201 |
| Asymmetric halo-Mannich-type reaction provides access to pyrrolidines and beta-proline derivatives. | The Journal of organic chemistry 20050916 |
| Atmospheric chemistry of C3-C6 cycloalkanecarbaldehydes. | The journal of physical chemistry. A 20050616 |
| New asymmetric halo aldol reaction provides a novel approach to biologically important chiral cyclothers and cycloamines. | Organic letters 20040610 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.