200,000+ products from a single source!
sales@angenechem.com
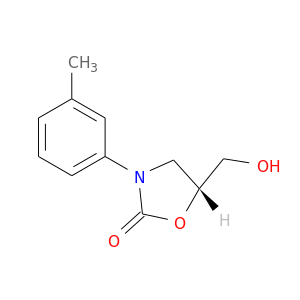
148278-79-9 | 2-Oxazolidinone, 5-(hydroxymethyl)-3-(3-methylphenyl)-, (5R)-
CAS No: 148278-79-9 Catalog No: AG001FKX MDL No:
Product Description
Catalog Number:
AG001FKX
Chemical Name:
2-Oxazolidinone, 5-(hydroxymethyl)-3-(3-methylphenyl)-, (5R)-
CAS Number:
148278-79-9
Molecular Formula:
C11H13NO3
Molecular Weight:
207.2258
IUPAC Name:
5-(hydroxymethyl)-3-(3-methylphenyl)-1,3-oxazolidin-2-one
InChI:
InChI=1S/C11H13NO3/c1-8-3-2-4-9(5-8)12-6-10(7-13)15-11(12)14/h2-5,10,13H,6-7H2,1H3
InChI Key:
MXUNKHLAEDCYJL-UHFFFAOYSA-N
SMILES:
OC[C@@H]1OC(=O)N(C1)c1cccc(c1)C
EC Number:
249-522-2
Properties
Complexity:
244
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
207.09g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
207.229g/mol
Monoisotopic Mass:
207.09g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
49.8A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| Recent development of potent analogues of oxazolidinone antibacterial agents. | Bioorganic & medicinal chemistry 20130201 |
| Novel reversible monoamine oxidase A inhibitors: highly potent and selective 3-(1H-pyrrol-3-yl)-2-oxazolidinones. | Journal of medicinal chemistry 20111208 |
| Development of a list of potentially inappropriate drugs for the korean elderly using the delphi method. | Healthcare informatics research 20101201 |
| Analysis of activity and inhibition of oxygen-dependent enzymes by optical respirometry on the LightCycler system. | Analytical biochemistry 20100215 |
| Mining biologically-active molecules for inhibitors of fatty acid amide hydrolase (FAAH): identification of phenmedipham and amperozide as FAAH inhibitors. | Bioorganic & medicinal chemistry letters 20091201 |
| Synthesis, molecular modeling studies and selective inhibitory activity against MAO of N1-propanoyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives. | European journal of medicinal chemistry 20081001 |
| Quantification of tricyclic antidepressants and monoamine oxidase inhibitors by high-performance liquid chromatography-tandem mass spectrometry in whole blood. | Journal of analytical toxicology 20070501 |
| Monoamine oxidase isoform-dependent tautomeric influence in the recognition of 3,5-diaryl pyrazole inhibitors. | Journal of medicinal chemistry 20070208 |
| Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study. | Journal of natural products 20060601 |
| Neuroendocrine predictors of the evolution of depression. | Dialogues in clinical neuroscience 20050901 |
| Design, synthesis, and biological activities of pyrrolylethanoneamine derivatives, a novel class of monoamine oxidases inhibitors. | Journal of medicinal chemistry 20050630 |
| 3-(1H-pyrrol-2-yl)-2-oxazolidinones as novel monoamine oxidase type A inhibitors. | Medicinal chemistry (Shariqah (United Arab Emirates)) 20050301 |
| SL25.1131 [3(S),3a(S)-3-methoxymethyl-7-[4,4,4-trifluorobutoxy]-3,3a,4,5-tetrahydro-1,3-oxazolo[3,4-a]quinolin-1-one], a new, reversible, and mixed inhibitor of monoamine oxidase-A and monoamine oxidase-B: biochemical and behavioral profile. | The Journal of pharmacology and experimental therapeutics 20040901 |
| A high-performance liquid chromatography method with photodiode-array UV detection for therapeutic drug monitoring of the nontricyclic antidepressant drugs. | Therapeutic drug monitoring 20031001 |
| Highly efficient CuI-catalyzed coupling of aryl bromides with oxazolidinones using Buchwald's protocol: a short route to linezolid and toloxatone. | Organic letters 20030403 |
| Synthesis and biological evaluation of enantiomerically pure pyrrolyl-oxazolidinones as a new class of potent and selective monoamine oxidase type A inhibitors. | Farmaco (Societa chimica italiana : 1989) 20030301 |
| 3-(1H-Pyrrol-1-yl)-2-oxazolidinones as reversible, highly potent, and selective inhibitors of monoamine oxidase type A. | Journal of medicinal chemistry 20020314 |
| The clinical pharmacology of depressive states. | Dialogues in clinical neuroscience 20020301 |
| Life-threatening pseudophaeochromocytoma after toloxatone, terbutaline, and phenylephrine. | Lancet (London, England) 19930227 |
| Comparison of the monoamine oxidase inhibiting properties of two reversible and selective monoamine oxidase-A inhibitors moclobemide and toloxatone, and assessment of their effect on psychometric performance in healthy subjects. | British journal of clinical pharmacology 19901201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.