200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 14795-83-6
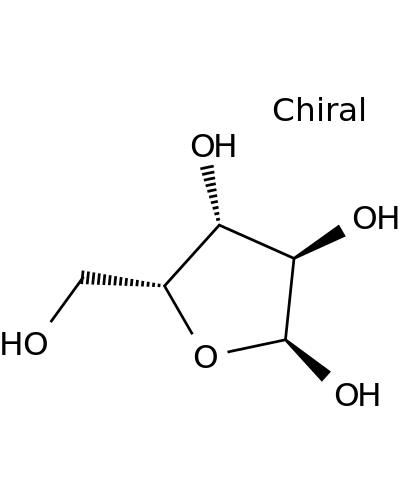
14795-83-6 | α-D-Xylofuranose
CAS No: 14795-83-6 Catalog No: AG001FBJ MDL No:
Product Description
Catalog Number:
AG001FBJ
Chemical Name:
α-D-Xylofuranose
CAS Number:
14795-83-6
Molecular Formula:
C5H10O5
Molecular Weight:
150.1299
IUPAC Name:
(2S,3R,4R,5R)-5-(hydroxymethyl)oxolane-2,3,4-triol
InChI:
InChI=1S/C5H10O5/c6-1-2-3(7)4(8)5(9)10-2/h2-9H,1H2/t2-,3+,4-,5+/m1/s1
InChI Key:
HMFHBZSHGGEWLO-LECHCGJUSA-N
SMILES:
OC[C@H]1O[C@@H]([C@@H]([C@H]1O)O)O
Properties
Complexity:
117
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
150.053g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
150.13g/mol
Monoisotopic Mass:
150.053g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
90.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2
Literature
| Title | Journal |
|---|---|
| Trans-α-xylosidase, a widespread enzyme activity in plants, introduces (1→4)-α-d-xylobiose side-chains into xyloglucan structures. | Phytochemistry 20120601 |
| First total synthesis of a naturally occurring iodinated 5'-deoxyxylofuranosyl marine nucleoside. | Marine drugs 20120401 |
| AtBGAL10 is the main xyloglucan β-galactosidase in Arabidopsis, and its absence results in unusual xyloglucan subunits and growth defects. | Plant physiology 20120301 |
| Isolation and structural characterisation of okara polysaccharides. | Molecules (Basel, Switzerland) 20120113 |
| Biology and pathogenesis of Acanthamoeba. | Parasites & vectors 20120101 |
| Beta-D-xylosidase from Selenomonas ruminantium: catalyzed reactions with natural and artificial substrates. | Applied biochemistry and biotechnology 20080301 |
| A one step synthetic approach to L-pyrimidine nucleosides using natural phosphate coated with potassium iodide as catalyst. | Nucleic acids symposium series (2004) 20080101 |
| Synthesis of highly condensed polycyclic carbohydrates by reaction of a spirocyclic enamino sulfonate derived from d-xylofuranose with bifunctional reagents. | The Journal of organic chemistry 20071207 |
| Probing the active site of Corynebacterium callunae starch phosphorylase through the characterization of wild-type and His334-->Gly mutant enzymes. | The FEBS journal 20071001 |
| SorF: a glycosyltransferase with promiscuous donor substrate specificity in vitro. | Chembiochem : a European journal of chemical biology 20070507 |
| Nucleoside synthesis from 3-alkylated sugars: role of 3beta-oxy substituents in directing nucleoside formation. | Organic & biomolecular chemistry 20060207 |
| Catalytic mechanism of alpha-retaining glucosyl transfer by Corynebacterium callunae starch phosphorylase: the role of histidine-334 examined through kinetic characterization of site-directed mutants. | The Biochemical journal 20050415 |
| Water T2 relaxation in sugar solutions. | Carbohydrate research 20050411 |
| Crystallization and preliminary X-ray analysis of alpha-xylosidase from Escherichia coli. | Acta crystallographica. Section F, Structural biology and crystallization communications 20050201 |
| Formation of a stable 14-helix in short oligomers of furanoid cis-beta-sugar-amino acid. | Journal of the American Chemical Society 20041027 |
| Overexpression and characterization of two unknown proteins, YicI and YihQ, originated from Escherichia coli. | Protein expression and purification 20040901 |
| Enzymatic synthesis of a library of beta-(1-->4) hetero- D-glucose and D-xylose-based oligosaccharides employing cellodextrin phosphorylase. | Carbohydrate research 20030910 |
| Efficient nitrogen alkylation with carbohydrates. | Carbohydrate research 20030523 |
| Alpha-glucosidase mutant catalyzes 'alpha-glycosynthase'-type reaction. | Bioscience, biotechnology, and biochemistry 20020401 |
| Modular furanoside phosphite ligands for asymmetric Pd-catalyzed allylic substitution. | The Journal of organic chemistry 20011228 |
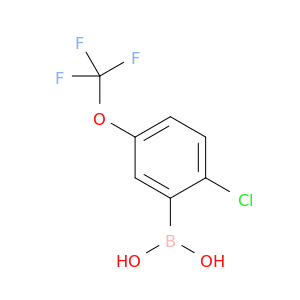
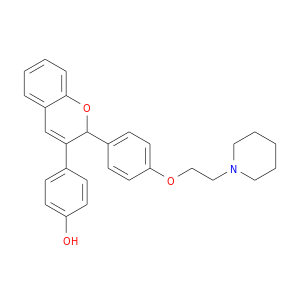
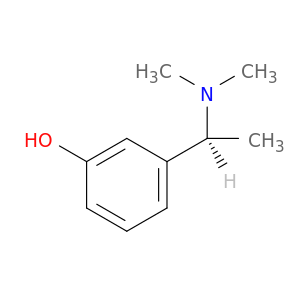
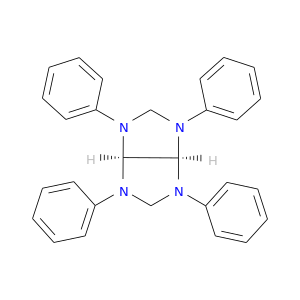
Related Products
© 2019 Angene International Limited. All rights Reserved.