200,000+ products from a single source!
sales@angenechem.com
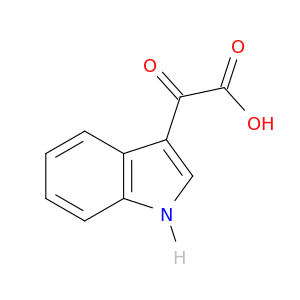
1477-49-2 | 1H-Indole-3-acetic acid, α-oxo-
CAS No: 1477-49-2 Catalog No: AG001F44 MDL No:MFCD00005625
Product Description
Catalog Number:
AG001F44
Chemical Name:
1H-Indole-3-acetic acid, α-oxo-
CAS Number:
1477-49-2
Molecular Formula:
C10H7NO3
Molecular Weight:
189.1675
MDL Number:
MFCD00005625
IUPAC Name:
2-(1H-indol-3-yl)-2-oxoacetic acid
InChI:
InChI=1S/C10H7NO3/c12-9(10(13)14)7-5-11-8-4-2-1-3-6(7)8/h1-5,11H,(H,13,14)
InChI Key:
DWLVFWDCSFTDOD-UHFFFAOYSA-N
SMILES:
OC(=O)C(=O)c1c[nH]c2c1cccc2
EC Number:
216-029-9
NSC Number:
71954
Properties
Complexity:
264
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
189.043g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
189.17g/mol
Monoisotopic Mass:
189.043g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
70.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.9
Literature
| Title | Journal |
|---|---|
| Zorrimidazolone, a bioactive alkaloid from the non-indigenous mediterranean stolidobranch Polyandrocarpa zorritensis. | Marine drugs 20110101 |
| Synthetic analogs of indole-containing natural products as inhibitors of sortase A and isocitrate lyase. | Bioorganic & medicinal chemistry letters 20101201 |
| 1-Toluene-sulfonyl-3-[(3'-hydroxy-5'-substituted)-gamma-butyrolactone]-indoles: synthesis, COX-2 inhibition and anti-cancer activities. | Bioorganic & medicinal chemistry letters 20080101 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Complete and remarkable reversal of chemoselectivity in [4 + 2] cycloadditions involving electron-poor indoles as dienophiles. Diels-Alder versus hetero-Diels-Alder processes. | The Journal of organic chemistry 20031017 |
| Lasting chemiluminescence of 3-indoleglyoxylyl chloride and its enhancement. | Analytical sciences : the international journal of the Japan Society for Analytical Chemistry 20030101 |
| alpha-Ketocarboxylic acid-based inhibitors of protein tyrosine phosphatases. | Bioorganic & medicinal chemistry letters 20010723 |
Related Products
© 2019 Angene International Limited. All rights Reserved.