200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 147664-41-3
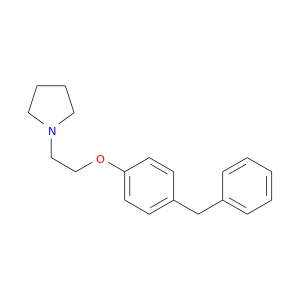
147664-41-3 | Pyrrolidine, 1-[2-[4-(phenylmethyl)phenoxy]ethyl]-
CAS No: 147664-41-3 Catalog No: AG001F2S MDL No:
Product Description
Catalog Number:
AG001F2S
Chemical Name:
Pyrrolidine, 1-[2-[4-(phenylmethyl)phenoxy]ethyl]-
CAS Number:
147664-41-3
Molecular Formula:
C19H23NO
Molecular Weight:
281.3920
IUPAC Name:
1-[2-(4-benzylphenoxy)ethyl]pyrrolidine
InChI:
InChI=1S/C19H23NO/c1-2-6-17(7-3-1)16-18-8-10-19(11-9-18)21-15-14-20-12-4-5-13-20/h1-3,6-11H,4-5,12-16H2
InChI Key:
JQTXWEHBUDESJC-UHFFFAOYSA-N
SMILES:
c1ccc(cc1)Cc1ccc(cc1)OCCN1CCCC1
Properties
Complexity:
273
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
281.178g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
281.399g/mol
Monoisotopic Mass:
281.178g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
12.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.3
Literature
| Title | Journal |
|---|---|
| Molecular characterization of the microsomal tamoxifen binding site. | The Journal of biological chemistry 20040806 |
| Synthesis of imidazopyridines and purines as potent inhibitors of leukotriene A4 hydrolase. | Bioorganic & medicinal chemistry letters 20030324 |
| Pyrrolidine and piperidine analogues of SC-57461A as potent, orally active inhibitors of leukotriene A(4) hydrolase. | Bioorganic & medicinal chemistry letters 20021202 |
| Structure-activity relationship studies on 1-[2-(4-Phenylphenoxy)ethyl]pyrrolidine (SC-22716), a potent inhibitor of leukotriene A(4) (LTA(4)) hydrolase. | Journal of medicinal chemistry 20000224 |
Related Products
© 2019 Angene International Limited. All rights Reserved.