200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 14700-62-0
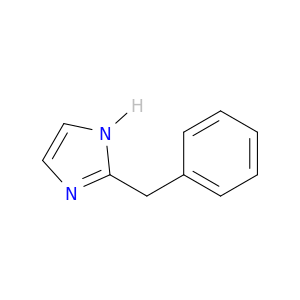
14700-62-0 | 1H-Imidazole, 2-(phenylmethyl)-
CAS No: 14700-62-0 Catalog No: AG001EHR MDL No:MFCD20488764
Product Description
Catalog Number:
AG001EHR
Chemical Name:
1H-Imidazole, 2-(phenylmethyl)-
CAS Number:
14700-62-0
Molecular Formula:
C10H10N2
Molecular Weight:
158.1998
MDL Number:
MFCD20488764
IUPAC Name:
2-benzyl-1H-imidazole
InChI:
InChI=1S/C10H10N2/c1-2-4-9(5-3-1)8-10-11-6-7-12-10/h1-7H,8H2,(H,11,12)
InChI Key:
NAPDOWNULRULLI-UHFFFAOYSA-N
SMILES:
c1ccc(cc1)Cc1ncc[nH]1
NSC Number:
46839
Properties
Complexity:
130
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
158.084g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
158.204g/mol
Monoisotopic Mass:
158.084g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
28.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2
Literature
| Title | Journal |
|---|---|
| Optimisation of imidazole compounds as selective TAAR1 agonists: discovery of RO5073012. | Bioorganic & medicinal chemistry letters 20120815 |
| Differential pharmacology and benefit/risk of azilsartan compared to other sartans. | Vascular health and risk management 20120101 |
| Phosphorylation State-Dependent High Throughput Screening of the c-Met Kinase. | Current chemical genomics 20100101 |
| Biological reactive intermediates that mediate dacarbazine cytotoxicity. | Cancer chemotherapy and pharmacology 20091201 |
| New potent imidazoisoquinolinone derivatives as anti-Trypanosoma cruzi agents: biological evaluation and structure-activity relationships. | Bioorganic & medicinal chemistry 20090215 |
| Air-stable, convenient to handle Pd based PEPPSI (pyridine enhanced precatalyst preparation, stabilization and initiation) themed precatalysts of N/O-functionalized N-heterocyclic carbenes and its utility in Suzuki-Miyaura cross-coupling reaction. | Dalton transactions (Cambridge, England : 2003) 20071028 |
| Induction of CYP1A and cyp2-mediated arachidonic acid epoxygenation and suppression of 20-hydroxyeicosatetraenoic acid by imidazole derivatives including the aromatase inhibitor vorozole. | Drug metabolism and disposition: the biological fate of chemicals 20060801 |
| H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. | Toxicology 20041015 |
| Induction of CYP1A by the N-imidazole derivative, 1-benzylimidazole. | Environmental toxicology and chemistry 20030401 |
| Exploration of the diaphorase activity of neutrophil NADPH oxidase. | European journal of biochemistry 20020201 |
| Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: glutathione conjugate formation. | Drug metabolism and disposition: the biological fate of chemicals 20011101 |
| Catechin metabolism: glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome p450. | Chemical research in toxicology 20010701 |
Related Products
© 2019 Angene International Limited. All rights Reserved.