200,000+ products from a single source!
sales@angenechem.com
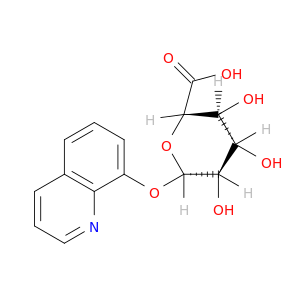
14683-61-5 | β-D-Glucopyranosiduronic acid, 8-quinolinyl
CAS No: 14683-61-5 Catalog No: AG001EDK MDL No:MFCD00063949
Product Description
Catalog Number:
AG001EDK
Chemical Name:
β-D-Glucopyranosiduronic acid, 8-quinolinyl
CAS Number:
14683-61-5
Molecular Formula:
C15H15NO7
Molecular Weight:
321.2821
MDL Number:
MFCD00063949
IUPAC Name:
(2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-quinolin-8-yloxyoxane-2-carboxylic acid
InChI:
InChI=1S/C15H15NO7/c17-10-11(18)13(14(20)21)23-15(12(10)19)22-8-5-1-3-7-4-2-6-16-9(7)8/h1-6,10-13,15,17-19H,(H,20,21)/t10-,11-,12+,13-,15+/m0/s1
InChI Key:
DPEGQJDYRIQRHI-DKBOKBLXSA-N
SMILES:
OC(=O)[C@H]1O[C@@H](Oc2cccc3c2nccc3)[C@@H]([C@H]([C@@H]1O)O)O
EC Number:
238-724-6
Properties
Complexity:
434
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
321.085g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
321.285g/mol
Monoisotopic Mass:
321.085g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
129A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.3
Literature
| Title | Journal |
|---|---|
| Enzymatic synthesis of (125/131)I labeled 8-hydroxyquinoline glucuronide and in vitro/in vivo evaluation of biological influence. | Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine 20110201 |
| Improved specific biodetection with ion trap mobility spectrometry (ITMS): a 10-min, multiplexed, immunomagnetic ELISA. | Analytical chemistry 20091215 |
| The in vitro metabolism of irinotecan (CPT-11) by carboxylesterase and beta-glucuronidase in human colorectal tumours. | British journal of clinical pharmacology 20060701 |
| Effective on-line purification for cationic compounds in rat bile using a column-switching LC technique. | Journal of pharmaceutical and biomedical analysis 20060213 |
| Cloning and expression of a rat liver phenobarbital-inducible UDP-glucuronosyltransferase (2B12) with specificity for monoterpenoid alcohols. | Archives of biochemistry and biophysics 19951001 |
| Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. | Drug metabolism and disposition: the biological fate of chemicals 19940101 |
| Screening for new compounds with antiherpes activity. | Antiviral research 19841001 |
Related Products
© 2019 Angene International Limited. All rights Reserved.