200,000+ products from a single source!
sales@angenechem.com
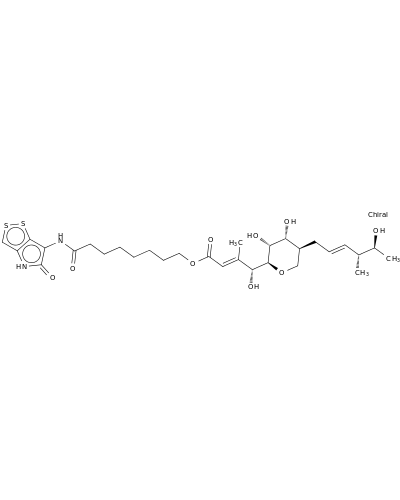
146697-04-3 | L-glycero-D-altro-Non-2-enonic acid, 5,9-anhydro-2,3,8-trideoxy-8-[(2E,4R,5S)-5-hydroxy-4-methyl-2-hexen-1-yl]-3-methyl-, 8-[(4,5-dihydro-5-oxo-1,2-dithiolo[4,3-b]pyrrol-6-yl)amino]-8-oxooctyl ester, (2E)-
CAS No: 146697-04-3 Catalog No: AG001E86 MDL No:MFCD00915727
Product Description
Catalog Number:
AG001E86
Chemical Name:
L-glycero-D-altro-Non-2-enonic acid, 5,9-anhydro-2,3,8-trideoxy-8-[(2E,4R,5S)-5-hydroxy-4-methyl-2-hexen-1-yl]-3-methyl-, 8-[(4,5-dihydro-5-oxo-1,2-dithiolo[4,3-b]pyrrol-6-yl)amino]-8-oxooctyl ester, (2E)-
CAS Number:
146697-04-3
Molecular Formula:
C30H44N2O9S2
Molecular Weight:
640.8084
MDL Number:
MFCD00915727
IUPAC Name:
[8-oxo-8-[(5-oxo-4H-dithiolo[4,3-b]pyrrol-6-yl)amino]octyl] (E)-4-[3,4-dihydroxy-5-[(E)-5-hydroxy-4-methylhex-2-enyl]oxan-2-yl]-4-hydroxy-3-methylbut-2-enoate
InChI:
InChI=1S/C30H44N2O9S2/c1-17(19(3)33)10-9-11-20-15-41-28(27(38)26(20)37)25(36)18(2)14-23(35)40-13-8-6-4-5-7-12-22(34)32-24-29-21(16-42-43-29)31-30(24)39/h9-10,14,16-17,19-20,25-28,33,36-38H,4-8,11-13,15H2,1-3H3,(H,31,39)(H,32,34)/b10-9+,18-14+
InChI Key:
JIEMCPGFAXNCQW-BFHPBESDSA-N
SMILES:
C/C(=C\C(=O)OCCCCCCCC(=O)Nc1c2sscc2[nH]c1=O)/[C@H]([C@@H]1OC[C@@H]([C@H]([C@H]1O)O)C/C=C/[C@H]([C@@H](O)C)C)O
Properties
Complexity:
1120
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
2
Exact Mass:
640.249g/mol
Formal Charge:
0
Heavy Atom Count:
43
Hydrogen Bond Acceptor Count:
11
Hydrogen Bond Donor Count:
6
Isotope Atom Count:
0
Molecular Weight:
640.807g/mol
Monoisotopic Mass:
640.249g/mol
Rotatable Bond Count:
17
Topological Polar Surface Area:
225A^2
Undefined Atom Stereocenter Count:
7
Undefined Bond Stereocenter Count:
0
XLogP3:
2
Literature
| Title | Journal |
|---|---|
| Enzymatic basis of 'hybridity' in thiomarinol biosynthesis. | Angewandte Chemie (International ed. in English) 20150420 |
| Engineered thiomarinol antibiotics active against MRSA are generated by mutagenesis and mutasynthesis of Pseudoalteromonas SANK73390. | Angewandte Chemie (International ed. in English) 20110328 |
| Synthesis and preliminary antibacterial evaluation of simplified thiomarinol analogs. | Bioorganic & medicinal chemistry 20090201 |
| Catalytic asymmetric synthesis of a potent thiomarinol antibiotic. | Journal of the American Chemical Society 20050216 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







