200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 146445-96-7
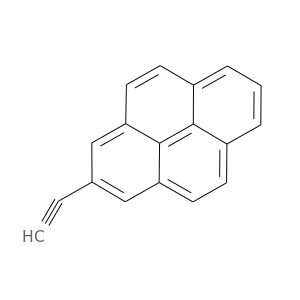
146445-96-7 | Pyrene, 2-ethynyl-
CAS No: 146445-96-7 Catalog No: AG001E0P MDL No:
Product Description
Catalog Number:
AG001E0P
Chemical Name:
Pyrene, 2-ethynyl-
CAS Number:
146445-96-7
Molecular Formula:
C18H10
Molecular Weight:
226.2720
IUPAC Name:
2-ethynylpyrene
InChI:
InChI=1S/C18H10/c1-2-12-10-15-8-6-13-4-3-5-14-7-9-16(11-12)18(15)17(13)14/h1,3-11H
InChI Key:
VVMDWPNNECNGTO-UHFFFAOYSA-N
SMILES:
C#Cc1cc2ccc3c4c2c(c1)ccc4ccc3
Properties
Complexity:
347
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
226.078g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
0
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
226.278g/mol
Monoisotopic Mass:
226.078g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
0A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5.3
Literature
| Title | Journal |
|---|---|
| Photobiological activity of Ru(II) dyads based on (pyren-1-yl)ethynyl derivatives of 1,10-phenanthroline. | Inorganic chemistry 20100315 |
| Reverse type I binding spectra of human cytochrome P450 1B1 induced by flavonoid, stilbene, pyrene, naphthalene, phenanthrene, and biphenyl derivatives that inhibit catalytic activity: a structure-function relationship study. | Chemical research in toxicology 20090701 |
| 1-, 2-, and 4-ethynylpyrenes in the structure of twisted intercalating nucleic acids: structure, thermal stability, and fluorescence relationship. | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Ruthenium-terpyridine complexes with multiple ethynylpyrenyl or ethynyltoluyl subunits: X-ray structure, redox, and spectroscopic properties. | Inorganic chemistry 20070903 |
| Different mechanisms for inhibition of human cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic inhibitors. | Chemical research in toxicology 20070301 |
| Cytochrome p450 enzymes mechanism based inhibitors: common sub-structures and reactivity. | Current drug metabolism 20051001 |
| Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. | Mutation research 20030101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.