200,000+ products from a single source!
sales@angenechem.com
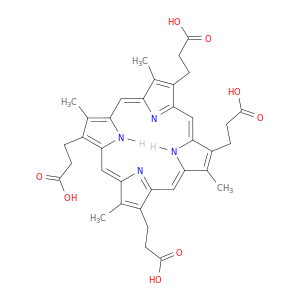
14643-66-4 | 21H,23H-Porphine-2,7,12,18-tetrapropanoic acid, 3,8,13,17-tetramethyl-
CAS No: 14643-66-4 Catalog No: AG001E1A MDL No:
Product Description
Catalog Number:
AG001E1A
Chemical Name:
21H,23H-Porphine-2,7,12,18-tetrapropanoic acid, 3,8,13,17-tetramethyl-
CAS Number:
14643-66-4
Molecular Formula:
C36H38N4O8
Molecular Weight:
654.7089
IUPAC Name:
3-[8,13,18-tris(2-carboxyethyl)-3,7,12,17-tetramethyl-21,24-dihydroporphyrin-2-yl]propanoic acid
InChI:
InChI=1S/C36H38N4O8/c1-17-21(5-9-33(41)42)29-14-27-19(3)22(6-10-34(43)44)30(39-27)15-28-20(4)24(8-12-36(47)48)32(40-28)16-31-23(7-11-35(45)46)18(2)26(38-31)13-25(17)37-29/h13-16,38,40H,5-12H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48)
InChI Key:
XNBNKCLBGTWWSD-UHFFFAOYSA-N
SMILES:
OC(=O)CCC1=C(C)C2=Cc3[nH]c(c(c3CCC(=O)O)C)C=C3N=C(C=c4[nH]c(=CC1=N2)c(C)c4CCC(=O)O)C(=C3C)CCC(=O)O
Properties
Complexity:
1170
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
654.269g/mol
Formal Charge:
0
Heavy Atom Count:
48
Hydrogen Bond Acceptor Count:
10
Hydrogen Bond Donor Count:
6
Isotope Atom Count:
0
Molecular Weight:
654.72g/mol
Monoisotopic Mass:
654.269g/mol
Rotatable Bond Count:
12
Topological Polar Surface Area:
207A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.6
Literature
| Title | Journal |
|---|---|
| ATP-dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity. | The Journal of biological chemistry 20120413 |
| Comparative study of the bactericidal effects of 5-aminolevulinic acid with blue and red light on Propionibacterium acnes. | The Journal of dermatology 20110701 |
| Tetrapyrrole binding affinity of the murine and human p22HBP heme-binding proteins. | Journal of molecular graphics & modelling 20101101 |
| Identification of tetrapyrrole compounds excreted by Rhodobacter sphaeroides and sources of the methyl hydrogens of bacteriochlorophyll a biosynthesized by R. sphaeroides, based on 13C-NMR spectral analysis of coproporphyrin III tetramethyl ester. | Chemical & pharmaceutical bulletin 20070701 |
| [Porphyrins as the early biomarkers for arsenic exposure of human]. | Huan jing ke xue= Huanjing kexue 20070501 |
| In vivo porphyrin production by P. acnes in untreated acne patients and its modulation by acne treatment. | Acta dermato-venereologica 20060101 |
| Inducing coproporphyria in rat hepatocyte cultures using cyclic AMP and cyclic AMP-releasing agents. | Archives of toxicology 20050701 |
| Effect of insulin and glucagon on accumulation of uroporphyrin and coproporphyrin from 5-aminolevulinate in hepatocyte cultures. | Archives of biochemistry and biophysics 20050701 |
| Porphyrins as early biomarkers for arsenic exposure in animals and humans. | Cellular and molecular biology (Noisy-le-Grand, France) 20021201 |
| Molecular, immunological, enzymatic and biochemical studies of coproporphyrinogen oxidase deficiency in a family with hereditary coproporphyria. | Cellular and molecular biology (Noisy-le-Grand, France) 20020201 |
| Roles of reactive oxygen species in monocyte activation induced by photochemical reactions during photodynamic therapy. | Frontiers of medical and biological engineering : the international journal of the Japan Society of Medical Electronics and Biological Engineering 20020101 |
| Neonatal-onset hereditary coproporphyria with male pseudohermaphrodism. | Blood 20011215 |
| Stacking and separation of coproporphyrin isomers by acetonitrile-salt mixtures in micellar electrokinetic chromatography. | Electrophoresis 20010701 |
Related Products
© 2019 Angene International Limited. All rights Reserved.