200,000+ products from a single source!
sales@angenechem.com
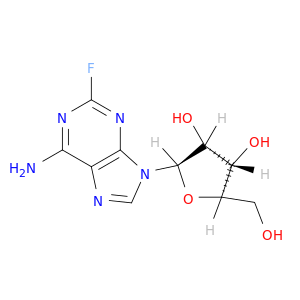
146-78-1 | Adenosine, 2-fluoro-
CAS No: 146-78-1 Catalog No: AG001DM5 MDL No:MFCD00866394
Product Description
Catalog Number:
AG001DM5
Chemical Name:
Adenosine, 2-fluoro-
CAS Number:
146-78-1
Molecular Formula:
C10H12FN5O4
Molecular Weight:
285.2318
MDL Number:
MFCD00866394
IUPAC Name:
(2R,3R,4S,5R)-2-(6-amino-2-fluoropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
InChI:
InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1
InChI Key:
HBUBKKRHXORPQB-UUOKFMHZSA-N
SMILES:
OC[C@H]1O[C@H]([C@@H]([C@@H]1O)O)n1cnc2c1nc(F)nc2N
UNII:
0S67290CRJ
Properties
Complexity:
367
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
285.087g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
9
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
285.235g/mol
Monoisotopic Mass:
285.087g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
140A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.6
Literature
| Title | Journal |
|---|---|
| Insights into phosphate cooperativity and influence of substrate modifications on binding and catalysis of hexameric purine nucleoside phosphorylases. | PloS one 20120101 |
| In vitro assessment of anticryptosporidial efficacy and cytotoxicity of adenosine analogues using a SYBR Green real-time PCR method. | The Journal of antimicrobial chemotherapy 20110301 |
| [The preparative method for 2-fluoroadenosine synthesis]. | Bioorganicheskaia khimiia 20090101 |
| Characterization of an engineered human purine nucleoside phosphorylase fused to an anti-her2/neu single chain Fv for use in ADEPT. | Journal of experimental & clinical cancer research : CR 20090101 |
| Crystal structures of Mycobacterium tuberculosis S-adenosyl-L-homocysteine hydrolase in ternary complex with substrate and inhibitors. | Protein science : a publication of the Protein Society 20081201 |
| High resolution crystal structures of Mycobacterium tuberculosis adenosine kinase: insights into the mechanism and specificity of this novel prokaryotic enzyme. | The Journal of biological chemistry 20070914 |
| [(3)H]Adenine is a suitable radioligand for the labeling of G protein-coupled adenine receptors but shows high affinity to bacterial contaminations in buffer solutions. | Purinergic signalling 20070901 |
| Nicotinamide 2-fluoroadenine dinucleotide unmasks the NAD+ glycohydrolase activity of Aplysia californica adenosine 5'-diphosphate ribosyl cyclase. | Biochemistry 20070403 |
| Biodistribution and PET imaging of [(18)F]-fluoroadenosine derivatives. | Nuclear medicine and biology 20070401 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Identification of a subversive substrate of Trichomonas vaginalis purine nucleoside phosphorylase and the crystal structure of the enzyme-substrate complex. | The Journal of biological chemistry 20050610 |
| Partial agonists for A(3) adenosine receptors. | Current topics in medicinal chemistry 20040101 |
| Stereoselective synthesis and antiviral activity of D-2',3'-didehydro-2',3'-dideoxy-2'-fluoro-4'-thionucleosides. | Journal of medicinal chemistry 20021024 |
| Structural determinants of A(3) adenosine receptor activation: nucleoside ligands at the agonist/antagonist boundary. | Journal of medicinal chemistry 20020926 |
| Purine analogs as potential anticytomegalovirus agents. | Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 19690901 |
Related Products
© 2019 Angene International Limited. All rights Reserved.