200,000+ products from a single source!
sales@angenechem.com
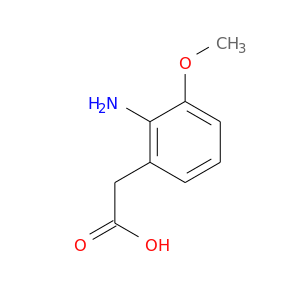
Home > Other Building Blocks > 145929-30-2
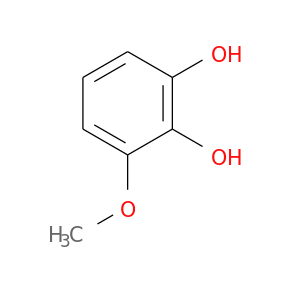
145929-30-2 | Benzenediol, methoxy- (9CI)
CAS No: 145929-30-2 Catalog No: AG001DID MDL No:
Product Description
Catalog Number:
AG001DID
Chemical Name:
Benzenediol, methoxy- (9CI)
CAS Number:
145929-30-2
Molecular Formula:
C7H8O3
Molecular Weight:
140.1366
IUPAC Name:
3-methoxybenzene-1,2-diol
InChI:
InChI=1S/C7H8O3/c1-10-6-4-2-3-5(8)7(6)9/h2-4,8-9H,1H3
InChI Key:
LPYUENQFPVNPHY-UHFFFAOYSA-N
SMILES:
COc1cccc(c1O)O
EC Number:
213-276-4
UNII:
IC13U5393C
NSC Number:
66525
Properties
Complexity:
105
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
140.047g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
140.138g/mol
Monoisotopic Mass:
140.047g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
49.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.8
Literature
| Title | Journal |
|---|---|
| Selective lignin and polysaccharide removal in natural fungal decay of wood as evidenced by in situ structural analyses. | Environmental microbiology 20110101 |
| Oxidative stress and vascular function: implications for pharmacologic treatments. | Current hypertension reports 20100601 |
| A previously uncultured, paper mill Propionibacterium is able to degrade O-aryl alkyl ethers and various aromatic hydrocarbons. | Chemosphere 20090601 |
| Urine biomarkers of risk in the molecular etiology of breast cancer. | Breast cancer : basic and clinical research 20090101 |
| Prevention of estrogen-DNA adduct formation in MCF-10F cells by resveratrol. | Free radical biology & medicine 20080715 |
| A novel catechol-based universal support for oligonucleotide synthesis. | The Journal of organic chemistry 20071221 |
| The reactivity of ortho-methoxy-substituted catechol radicals with sulfhydryl groups: contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. | Biochemical pharmacology 20070801 |
| The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. | Archives of biochemistry and biophysics 20070115 |
| Alanine 101 and alanine 110 of the alpha subunit of Pseudomonas stutzeri OX1 toluene-o-xylene monooxygenase influence the regiospecific oxidation of aromatics. | Biotechnology and bioengineering 20051205 |
| Quantitative structure toxicity relationships for catechols in isolated rat hepatocytes. | Chemico-biological interactions 20040415 |
| Vibrational analysis of substituted phenols: part I. Vibrational spectra, normal coordinate analysis and transferability of force constants of some formyl-, methoxy-, formylmethoxy-, methyl- and halogeno-phenols. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20021201 |
| In vitro activity of polyhydroxycarboxylates against herpesviruses and HIV. | Antiviral chemistry & chemotherapy 20011101 |
| Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. | Molecular pharmacology 20010201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.