200,000+ products from a single source!
sales@angenechem.com
Home > Pyridines > 145733-36-4
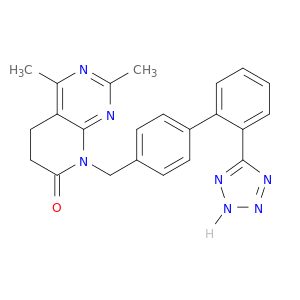
145733-36-4 | Pyrido[2,3-d]pyrimidin-7(6H)-one, 5,8-dihydro-2,4-dimethyl-8-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
CAS No: 145733-36-4 Catalog No: AG001DDC MDL No:MFCD00865905
Product Description
Catalog Number:
AG001DDC
Chemical Name:
Pyrido[2,3-d]pyrimidin-7(6H)-one, 5,8-dihydro-2,4-dimethyl-8-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
CAS Number:
145733-36-4
Molecular Formula:
C23H21N7O
Molecular Weight:
411.4591
MDL Number:
MFCD00865905
IUPAC Name:
2,4-dimethyl-8-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-5,6-dihydropyrido[2,3-d]pyrimidin-7-one
InChI:
InChI=1S/C23H21N7O/c1-14-18-11-12-21(31)30(23(18)25-15(2)24-14)13-16-7-9-17(10-8-16)19-5-3-4-6-20(19)22-26-28-29-27-22/h3-10H,11-13H2,1-2H3,(H,26,27,28,29)
InChI Key:
ADXGNEYLLLSOAR-UHFFFAOYSA-N
SMILES:
Cc1nc(C)c2c(n1)N(Cc1ccc(cc1)c1ccccc1c1nn[nH]n1)C(=O)CC2
UNII:
48G92V856H
Properties
Complexity:
625
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
411.181g/mol
Formal Charge:
0
Heavy Atom Count:
31
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
411.469g/mol
Monoisotopic Mass:
411.181g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
101A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3
Literature
| Title | Journal |
|---|---|
| Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. | Chemical research in toxicology 20170515 |
| Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps). | PLoS computational biology 20111201 |
| Angiotensin II receptor type 1 (AT1) selective nonpeptidic antagonists--a perspective. | Bioorganic & medicinal chemistry 20101215 |
| A predictive ligand-based Bayesian model for human drug-induced liver injury. | Drug metabolism and disposition: the biological fate of chemicals 20101201 |
| Pharmacophore, drug metabolism, and pharmacokinetics models on non-peptide AT1, AT2, and AT1/AT2 angiotensin II receptor antagonists. | Journal of medicinal chemistry 20050630 |
| Angiotensin II AT1 receptor antagonists. Clinical implications of active metabolites. | Journal of medicinal chemistry 20030605 |
| Novel human metabolites of the angiotensin-II antagonist tasosartan and their pharmacological effects. | Bioorganic & medicinal chemistry letters 20020805 |
| Pharmacology of AT1-receptor blockers. | Blood pressure. Supplement 20010101 |
| Tasosartan, enoltasosartan, and angiotensin II receptor blockade: the confounding role of protein binding. | The Journal of pharmacology and experimental therapeutics 20001101 |
| Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy. | Journal of medicinal chemistry 19960202 |
Related Products
© 2019 Angene International Limited. All rights Reserved.