200,000+ products from a single source!
sales@angenechem.com
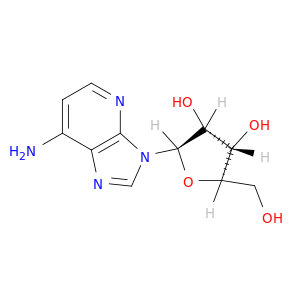
14432-09-8 | 3H-Imidazo[4,5-b]pyridin-7-amine, 3-β-D-ribofuranosyl-
CAS No: 14432-09-8 Catalog No: AG001JBZ MDL No:
Product Description
Catalog Number:
AG001JBZ
Chemical Name:
3H-Imidazo[4,5-b]pyridin-7-amine, 3-β-D-ribofuranosyl-
CAS Number:
14432-09-8
Molecular Formula:
C11H14N4O4
Molecular Weight:
266.2533
IUPAC Name:
(2R,3R,4S,5R)-2-(7-aminoimidazo[4,5-b]pyridin-3-yl)-5-(hydroxymethyl)oxolane-3,4-diol
InChI:
InChI=1S/C11H14N4O4/c12-5-1-2-13-10-7(5)14-4-15(10)11-9(18)8(17)6(3-16)19-11/h1-2,4,6,8-9,11,16-18H,3H2,(H2,12,13)/t6-,8-,9-,11-/m1/s1
InChI Key:
NVUDDRWKCUAERS-PNHWDRBUSA-N
SMILES:
OC[C@H]1O[C@H]([C@@H]([C@@H]1O)O)n1cnc2c1nccc2N
Properties
Complexity:
334
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
266.102g/mol
Formal Charge:
0
Heavy Atom Count:
19
Hydrogen Bond Acceptor Count:
7
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
266.257g/mol
Monoisotopic Mass:
266.102g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
127A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.7
Literature
| Title | Journal |
|---|---|
| Investigations into the origin of the molecular recognition of several adenosine deaminase inhibitors. | Journal of medicinal chemistry 20110113 |
| Conformational change of adenosine deaminase during ligand-exchange in a crystal. | Biochemical and biophysical research communications 20080815 |
| [Conformation of adenosine deaminase in complexes with inhibitors: application of selective quenching of fluorescence emission]. | Biofizika 20080101 |
| Binding thermodynamics of the transition state analogue coformycin and of the ground state analogue 1-deazaadenosine to bovine adenosine deaminase. | Journal of enzyme inhibition 20010101 |
| N-cycloalkyl derivatives of adenosine and 1-deazaadenosine as agonists and partial agonists of the A(1) adenosine receptor. | Journal of medicinal chemistry 20000127 |
| Anticancer and antiviral effects and inactivation of S-adenosyl-L-homocysteine hydrolase with 5'-carboxaldehydes and oximes synthesized from adenosine and sugar-modified analogues. | Journal of medicinal chemistry 19970523 |
| Synthesis and biological evaluation of N6-cycloalkyl derivatives of 1-deazaadenine nucleosides: a new class of anti-human immunodeficiency virus agents. | Journal of medicinal chemistry 19950929 |
Related Products
© 2019 Angene International Limited. All rights Reserved.