200,000+ products from a single source!
sales@angenechem.com
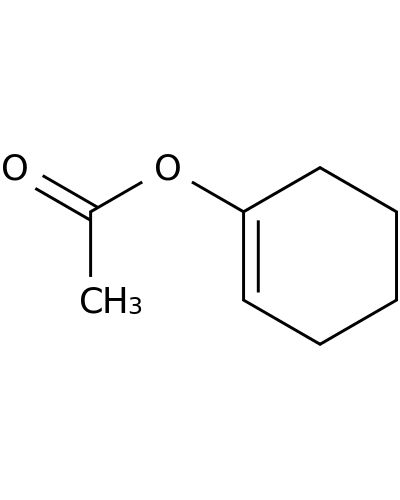
1424-22-2 | 1-CYCLOHEXENYL ACETATE
CAS No: 1424-22-2 Catalog No: AG003E95 MDL No:MFCD00013774
Product Description
Catalog Number:
AG003E95
Chemical Name:
1-CYCLOHEXENYL ACETATE
CAS Number:
1424-22-2
Molecular Formula:
C8H12O2
Molecular Weight:
140.1797
MDL Number:
MFCD00013774
IUPAC Name:
cyclohexen-1-yl acetate
InChI:
InChI=1S/C8H12O2/c1-7(9)10-8-5-3-2-4-6-8/h5H,2-4,6H2,1H3
InChI Key:
DRJNNZMCOCQJGI-UHFFFAOYSA-N
SMILES:
CC(=O)OC1=CCCCC1
EC Number:
215-838-4
UNII:
FHZ4PS0MLN
NSC Number:
18899
Properties
Complexity:
159
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
140.084g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
140.182g/mol
Monoisotopic Mass:
140.084g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
26.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Novel C2-symmetric planar chiral diphosphine ligands and their application in pd-catalyzed asymmetric allylic substitutions. | The Journal of organic chemistry 20070831 |
| SUPRAphos-based palladium catalysts for the kinetic resolution of racemic cyclohexenyl acetate. | Chemical communications (Cambridge, England) 20070614 |
| Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate. | Organic letters 20060622 |
| Palladium-catalyzed enantioselective allylic alkylation of thiocarboxylate ions: asymmetric synthesis of allylic thioesters and memory effect/dynamic kinetic resolution of allylic esters. | The Journal of organic chemistry 20040611 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







