200,000+ products from a single source!
sales@angenechem.com
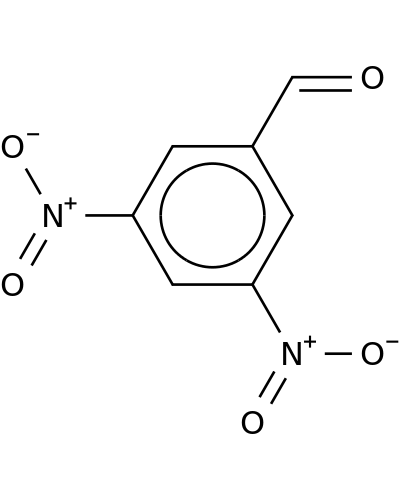
14193-18-1 | Benzaldehyde, 3,5-dinitro-
CAS No: 14193-18-1 Catalog No: AG001HEN MDL No:MFCD04971227
Product Description
Catalog Number:
AG001HEN
Chemical Name:
Benzaldehyde, 3,5-dinitro-
CAS Number:
14193-18-1
Molecular Formula:
C7H4N2O5
Molecular Weight:
196.1171
MDL Number:
MFCD04971227
IUPAC Name:
3,5-dinitrobenzaldehyde
InChI:
InChI=1S/C7H4N2O5/c10-4-5-1-6(8(11)12)3-7(2-5)9(13)14/h1-4H
InChI Key:
YCTNWTBGSOCMRO-UHFFFAOYSA-N
SMILES:
O=Cc1cc(cc(c1)[N+](=O)[O-])[N+](=O)[O-]
Properties
Complexity:
234
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
196.012g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
196.118g/mol
Monoisotopic Mass:
196.012g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
109A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Focused review: agmatine in fermented foods. | Frontiers in microbiology 20120101 |
| Study of stereoselective interactions of carbamoylated quinine and quinidine with 3,5-dinitrobenzoyl α-amino acids using VCD spectroscopy in the region of C−H stretching vibrations. | Chirality 20110401 |
| Polyamines in foods: development of a food database. | Food & nutrition research 20110101 |
| A 3,5-dinitro-benzoyl derivative of a stereoisomer of glycerol menthonide. | Acta crystallographica. Section E, Structure reports online 20090701 |
| (1RS,4RS,5RS)-Methyl 2-(3,5-dinitro-benzo-yl)-2-oxa-3-aza-bicyclo-[3.3.0]oct-7-ene-4-carboxyl-ate. | Acta crystallographica. Section E, Structure reports online 20090501 |
| Pi-Pi complexation of bupivacaine and analogues with aromatic receptors: implications for overdose remediation. | International journal of nanomedicine 20070901 |
| Preparation of a mercaptopropyl bonded silica intermediate in supercritical carbon dioxide: synthesis, characterisation and chromatography of a quinine based chiral stationary phase. | Journal of chromatography. A 20070713 |
| [Preparation and evaluation of 3,5-dinitrobenzoyl-bonded silica gel stationary phase for high performance liquid chromatography]. | Se pu = Chinese journal of chromatography 20050301 |
| Efficient resolution of racemic 1,1'-bi-2-naphthol with chiral selectors identified from a small library. | Journal of chromatography. A 20050107 |
| Determination of azide as the 3,5-dinitrobenzoyl derivative by capillary electrophoresis. | Journal of chromatography. A 20040806 |
| Retention of 3,5-dinitrobenzoyl derivatives of linear alcohol polyethoxylates in reversed-phase liquid chromatographic system with acetonitrile-water mobile phase. | Journal of chromatography. A 20031222 |
| Micro-HPLC and standard-size HPLC for the separation of peptide stereoisomers employing an ion-exchange principle. | Journal of pharmaceutical and biomedical analysis 20030115 |
| Enantiomeric separation of diuretics on a novel Pirkle-type chiral stationary phase. | Biomedical chromatography : BMC 20021001 |
| Enantiomeric separation of N-protected amino acids by non-aqueous capillary electrophoresis with dimeric forms of quinine and quinidine derivatives serving as chiral selectors. | Journal of chromatography. A 20020301 |
| Solid-phase synthesis of chiral stationary phases based on 2,4,5,6-tetrachloro-1,3-dicyanobenzene derivatives spaced from N-3,5-dinitrobenzoyl alpha-amino acids: comparative study of their resolution efficacy. | Chirality 20010601 |
| Synthesis of branched polysaccharides by polymerization of 6-O-t-butyldimethylsilyl-D-glucal through stereoregular haloglycosylation. | Carbohydrate letters 20010101 |
| Immobilization of difunctional building blocks on hydroxysuccinimide activated silica: versatile in situ preparation of chiral stationary phases. | Chirality 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.