200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 141636-65-9
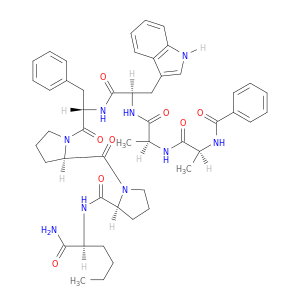
141636-65-9 | L-Norleucinamide, N-benzoyl-L-alanyl-L-alanyl-D-tryptophyl-L-phenylalanyl-D-prolyl-L-prolyl-
CAS No: 141636-65-9 Catalog No: AG001GRC MDL No:
Product Description
Catalog Number:
AG001GRC
Chemical Name:
L-Norleucinamide, N-benzoyl-L-alanyl-L-alanyl-D-tryptophyl-L-phenylalanyl-D-prolyl-L-prolyl-
CAS Number:
141636-65-9
Molecular Formula:
C49H61N9O8
Molecular Weight:
904.0641
IUPAC Name:
(2S)-N-[(2S)-1-amino-1-oxohexan-2-yl]-1-[(2R)-1-[(2S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-benzamidopropanoyl]amino]propanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-3-phenylpropanoyl]pyrrolidine-2-carbonyl]pyrrolidine-2-carboxamide
InChI:
InChI=1S/C49H61N9O8/c1-4-5-21-37(42(50)59)54-47(64)40-23-14-25-57(40)49(66)41-24-15-26-58(41)48(65)39(27-32-16-8-6-9-17-32)56-46(63)38(28-34-29-51-36-22-13-12-20-35(34)36)55-44(61)31(3)52-43(60)30(2)53-45(62)33-18-10-7-11-19-33/h6-13,16-20,22,29-31,37-41,51H,4-5,14-15,21,23-28H2,1-3H3,(H2,50,59)(H,52,60)(H,53,62)(H,54,64)(H,55,61)(H,56,63)/t30-,31-,37-,38+,39-,40-,41+/m0/s1
InChI Key:
CWEISCOCKRHHOU-UJYVDGJVSA-N
SMILES:
CCCC[C@@H](C(=O)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2c1cccc2)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1ccccc1)C)C
Properties
Complexity:
1720
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
7
Defined Bond Stereocenter Count:
0
Exact Mass:
903.464g/mol
Formal Charge:
0
Heavy Atom Count:
66
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
7
Isotope Atom Count:
0
Molecular Weight:
904.082g/mol
Monoisotopic Mass:
903.464g/mol
Rotatable Bond Count:
20
Topological Polar Surface Area:
245A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.4
Literature
| Title | Journal |
|---|---|
| Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. | British journal of pharmacology 20081201 |
| Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. | Journal of neurophysiology 19941001 |
| Synthesis and characterization of selective fluorescent ligands for the neurokinin NK2 receptor. | Journal of medicinal chemistry 19940624 |
| Highly potent and selective heptapeptide antagonists of the neurokinin NK-2 receptor. | Journal of medicinal chemistry 19920710 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.