200,000+ products from a single source!
sales@angenechem.com
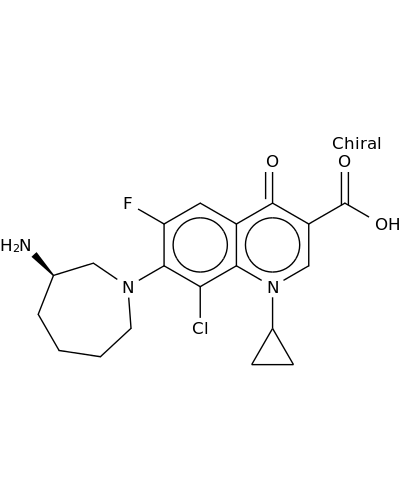
141388-76-3 | 3-Quinolinecarboxylic acid, 7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-
CAS No: 141388-76-3 Catalog No: AG001GBH MDL No:MFCD00917502
Product Description
Catalog Number:
AG001GBH
Chemical Name:
3-Quinolinecarboxylic acid, 7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-
CAS Number:
141388-76-3
Molecular Formula:
C19H21ClFN3O3
Molecular Weight:
393.8397
MDL Number:
MFCD00917502
IUPAC Name:
7-[(3R)-3-aminoazepan-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxoquinoline-3-carboxylic acid
InChI:
InChI=1S/C19H21ClFN3O3/c20-15-16-12(18(25)13(19(26)27)9-24(16)11-4-5-11)7-14(21)17(15)23-6-2-1-3-10(22)8-23/h7,9-11H,1-6,8,22H2,(H,26,27)/t10-/m1/s1
InChI Key:
QFFGVLORLPOAEC-SNVBAGLBSA-N
SMILES:
N[C@@H]1CCCCN(C1)c1c(F)cc2c(c1Cl)n(cc(c2=O)C(=O)O)C1CC1
UNII:
BFE2NBZ7NX
Properties
Complexity:
656
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
393.126g/mol
Formal Charge:
0
Heavy Atom Count:
27
Hydrogen Bond Acceptor Count:
7
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
393.843g/mol
Monoisotopic Mass:
393.126g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
86.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.1
Literature
| Title | Journal |
|---|---|
| Determination of enantiomeric impurity in besifloxacin hydrochloride by chiral high-performance liquid chromatography with precolumn derivatization. | Chirality 20120701 |
| Besifloxacin ophthalmic suspension, 0.6%: a novel topical fluoroquinolone for bacterial conjunctivitis. | Advances in therapy 20120601 |
| Besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis in adults and children. | Clinical drug investigation 20120501 |
| Microbiological etiology and susceptibility of bacterial conjunctivitis isolates from clinical trials with ophthalmic, twice-daily besifloxacin. | Advances in therapy 20120501 |
| Challenges in assessing microbial susceptibility and predicting clinical response to newer-generation fluoroquinolones. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20120201 |
| Acute and twenty-eight days repeated oral dose toxicity study of besifloxacin in Wistar albino rats. | Environmental toxicology and pharmacology 20110701 |
| Human aqueous humor concentrations of besifloxacin, moxifloxacin, and gatifloxacin after topical ocular application. | Journal of cataract and refractive surgery 20110601 |
| In vitro time-kill experiments with besifloxacin, moxifloxacin and gatifloxacin in the absence and presence of benzalkonium chloride. | The Journal of antimicrobial chemotherapy 20110401 |
| Evaluation of the effect of bacterial efflux pumps on the antibacterial activity of the novel fluoroquinolone besifloxacin. | Journal of chemotherapy (Florence, Italy) 20110401 |
| Comparison of besifloxacin, gatifloxacin, and moxifloxacin against strains of pseudomonas aeruginosa with different quinolone susceptibility patterns in a rabbit model of keratitis. | Cornea 20110101 |
| Relevance of aqueous humor concentrations of fluoroquinolones. | Journal of cataract and refractive surgery 20110101 |
| Efficacy and tolerability of besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study in adults and children. | Clinical therapeutics 20110101 |
| Topoisomerase mutations that are associated with high-level resistance to earlier fluoroquinolones in Staphylococcus aureus have less effect on the antibacterial activity of besifloxacin. | Chemotherapy 20110101 |
| Penetration and effectiveness of prophylactic fluoroquinolones in experimental methicillin-sensitive or methicillin-resistant Staphylococcus aureus anterior chamber infections. | Journal of cataract and refractive surgery 20101201 |
| An anterior chamber toxicity study evaluating Besivance, AzaSite, and Ciprofloxacin. | American journal of ophthalmology 20101001 |
| Ocular pharmacokinetics/pharmacodynamics of besifloxacin, moxifloxacin, and gatifloxacin following topical administration to pigmented rabbits. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20101001 |
| Aqueous penetration of moxifloxacin 0.5% ophthalmic solution and besifloxacin 0.6% ophthalmic suspension in cataract surgery patients. | Journal of cataract and refractive surgery 20100901 |
| Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenzae. | The Journal of antimicrobial chemotherapy 20100701 |
| Comparative effects of besifloxacin and other fluoroquinolones on corneal reepithelialization in the rabbit. | Journal of cataract and refractive surgery 20100601 |
| Comparative efficacy of besifloxacin and other fluoroquinolones in a prophylaxis model of penicillin-resistant Streptococcus pneumoniae rabbit endophthalmitis. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20100601 |
| Efficacy and safety of besifloxacin ophthalmic suspension 0.6% in children and adolescents with bacterial conjunctivitis: a post hoc, subgroup analysis of three randomized, double-masked, parallel-group, multicenter clinical trials. | Paediatric drugs 20100401 |
| Efficacy of besifloxacin in an early treatment model of methicillin-resistant Staphylococcus aureus keratitis. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20100401 |
| Besifloxacin: a topical fluoroquinolone for the treatment of bacterial conjunctivitis. | Clinical therapeutics 20100301 |
| New Drugs2010, PART 1. | Nursing 20100201 |
| Besifloxacin ophthalmic suspension 0.6%. | Drugs 20100101 |
| Safety and tolerability of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis: data from six clinical and phase I safety studies. | Clinical drug investigation 20100101 |
| Efficacy of besifloxacin in a rabbit model of methicillin-resistant Staphylococcus aureus keratitis. | Cornea 20091001 |
| Besifloxacin, a new ophthalmic fluoroquinolone for the treatment of bacterial conjunctivitis. | Expert opinion on pharmacotherapy 20091001 |
| Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. | Ophthalmology 20090901 |
| Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20090801 |
| Besifloxacin ophthalmic suspension for bacterial conjunctivitis. | Drugs of today (Barcelona, Spain : 1998) 20090801 |
| Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. | Current medical research and opinion 20090501 |
| Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. | The Journal of antimicrobial chemotherapy 20090301 |
| Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: A multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. | Clinical therapeutics 20090301 |
| New drugs: golimumab, besifloxacin hydrochloride, and artemether/lumefantrine. | Journal of the American Pharmacists Association : JAPhA 20090101 |
| Anti-inflammatory effects of besifloxacin, a novel fluoroquinolone, in primary human corneal epithelial cells. | Current eye research 20081101 |
| Quantitative determination of besifloxacin, a novel fluoroquinolone antimicrobial agent, in human tears by liquid chromatography-tandem mass spectrometry. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20080501 |
| Besifloxacin, a novel fluoroquinolone antimicrobial agent, exhibits potent inhibition of pro-inflammatory cytokines in human THP-1 monocytes. | The Journal of antimicrobial chemotherapy 20080101 |
| Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. | Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 20070601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.