200,000+ products from a single source!
sales@angenechem.com
Home > Fluorides > 140841-32-3
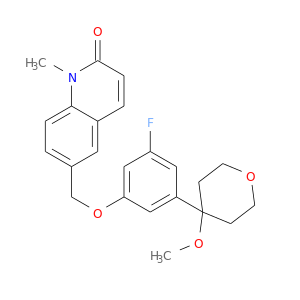
140841-32-3 | 2(1H)-Quinolinone, 6-[[3-fluoro-5-(tetrahydro-4-methoxy-2H-pyran-4-yl)phenoxy]methyl]-1-methyl-
CAS No: 140841-32-3 Catalog No: AG001CVW MDL No:
Product Description
Catalog Number:
AG001CVW
Chemical Name:
2(1H)-Quinolinone, 6-[[3-fluoro-5-(tetrahydro-4-methoxy-2H-pyran-4-yl)phenoxy]methyl]-1-methyl-
CAS Number:
140841-32-3
Molecular Formula:
C23H24FNO4
Molecular Weight:
397.4394
IUPAC Name:
6-[[3-fluoro-5-(4-methoxyoxan-4-yl)phenoxy]methyl]-1-methylquinolin-2-one
InChI:
InChI=1S/C23H24FNO4/c1-25-21-5-3-16(11-17(21)4-6-22(25)26)15-29-20-13-18(12-19(24)14-20)23(27-2)7-9-28-10-8-23/h3-6,11-14H,7-10,15H2,1-2H3
InChI Key:
SLZBEPLWGGRZAY-UHFFFAOYSA-N
SMILES:
COC1(CCOCC1)c1cc(OCc2ccc3c(c2)ccc(=O)n3C)cc(c1)F
UNII:
B6G6OX1ZH5
Properties
Complexity:
606
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
397.169g/mol
Formal Charge:
0
Heavy Atom Count:
29
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
397.446g/mol
Monoisotopic Mass:
397.169g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
48A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.9
Literature
| Title | Journal |
|---|---|
| Pharmacophore modeling and virtual screening for designing potential 5-lipoxygenase inhibitors. | Bioorganic & medicinal chemistry letters 20100201 |
| Indole derivatives as potent inhibitors of 5-lipoxygenase: design, synthesis, biological evaluation, and molecular modeling. | Bioorganic & medicinal chemistry letters 20070501 |
| New COX-2/5-LOX inhibitors: apoptosis-inducing agents potentially useful in prostate cancer chemotherapy. | Journal of medicinal chemistry 20041202 |
| Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin. | British journal of pharmacology 20030801 |
| Induction of hepatic microsomal drug-metabolizing enzymes by inhibitors of 5-lipoxygenase (5-LO): studies in rats and 5-LO knockout mice. | Toxicological sciences : an official journal of the Society of Toxicology 20010901 |
| Inhibition of 5-lipoxygenase diminishes neurally evoked tachykinergic contraction of guinea pig isolated airway. | The Journal of pharmacology and experimental therapeutics 19980501 |
| Characterization of the arachidonate and ATP binding sites of human 5-lipoxygenase using photoaffinity labeling and enzyme immobilization. | Biochemistry 19951017 |
| The 5-lipoxygenase inhibitors ZD2138 and ZM230487 are potent and selective inhibitors of several antigen-induced guinea-pig pulmonary responses. | European journal of pharmacology 19940523 |
| Calcium ionophore (A-23187) induced peritoneal eicosanoid biosynthesis: a rapid method to evaluate inhibitors of arachidonic acid metabolism in vivo. | Mediators of inflammation 19930101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







