200,000+ products from a single source!
sales@angenechem.com
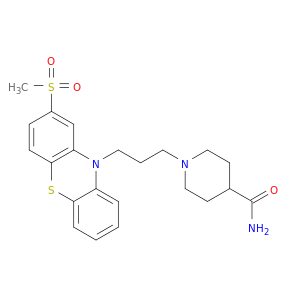
14008-44-7 | 4-Piperidinecarboxamide, 1-[3-[2-(methylsulfonyl)-10H-phenothiazin-10-yl]propyl]-
CAS No: 14008-44-7 Catalog No: AG001C10 MDL No:
Product Description
Catalog Number:
AG001C10
Chemical Name:
4-Piperidinecarboxamide, 1-[3-[2-(methylsulfonyl)-10H-phenothiazin-10-yl]propyl]-
CAS Number:
14008-44-7
Molecular Formula:
C22H27N3O3S2
Molecular Weight:
445.5981
IUPAC Name:
1-[3-(2-methylsulfonylphenothiazin-10-yl)propyl]piperidine-4-carboxamide
InChI:
InChI=1S/C22H27N3O3S2/c1-30(27,28)17-7-8-21-19(15-17)25(18-5-2-3-6-20(18)29-21)12-4-11-24-13-9-16(10-14-24)22(23)26/h2-3,5-8,15-16H,4,9-14H2,1H3,(H2,23,26)
InChI Key:
BQDBKDMTIJBJLA-UHFFFAOYSA-N
SMILES:
NC(=O)C1CCN(CC1)CCCN1c2ccccc2Sc2c1cc(cc2)S(=O)(=O)C
EC Number:
237-818-4
UNII:
238S75V9AV
Properties
Complexity:
703
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
445.149g/mol
Formal Charge:
0
Heavy Atom Count:
30
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
445.596g/mol
Monoisotopic Mass:
445.149g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
117A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.1
Literature
| Title | Journal |
|---|---|
| Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. | Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 20111001 |
| Fragmentation pathways of metopimazine and its metabolite using ESI-MS(n), HR-MS and H/D exchange. | Journal of mass spectrometry : JMS 20101001 |
| Development and validation of stability indicating HPLC and HPTLC methods for determination of sulpiride and mebeverine hydrochloride in combination. | European journal of medicinal chemistry 20100901 |
| Investigational use of metomidate hydrochloride as a shipping additive for two ornamental fishes. | Journal of aquatic animal health 20090901 |
| Effect of iontophoresis and penetration enhancers on transdermal absorption of metopimazine. | Journal of dermatological science 20081201 |
| Percutaneous absorption of metopimazine and effect of cyclodextrins. | Drug development and industrial pharmacy 20080501 |
| Optimizing antiemetic therapy in multiple-day and multiple cycles of chemotherapy. | Current opinion in supportive and palliative care 20080301 |
| [Tolerance and efficacy of mefloquine as the first line treatment of uncomplicated P. falciparum malaria in children]. | Pathologie-biologie 20080201 |
| Evidence of lowest brain penetration of an antiemetic drug, metopimazine, compared to domperidone, metoclopramide and chlorpromazine, using an in vitro model of the blood-brain barrier. | Pharmacological research 20070701 |
| Randomized, double-blind trial comparing the antiemetic effect of tropisetron plus metopimazine with tropisetron plus placebo in patients receiving multiple cycles of multiple-day cisplatin-based chemotherapy. | Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 20070401 |
| Differential pulse cathodic voltammetric determination of floctafenine and metopimazine. | Journal of pharmaceutical and biomedical analysis 20070312 |
| Efficacy and safety of artesunate plus amodiaquine in routine use for the treatment of uncomplicated malaria in Casamance, southern Sénégal. | Malaria journal 20070101 |
| Aprepitant: the evidence for its place in the prevention of chemotherapy-induced nausea and vomiting. | Core evidence 20070101 |
| A pilot study of ondansetron plus metopimazine vs. ondansetron monotherapy in children receiving highly emetogenic chemotherapy: a Bayesian randomized serial N-of-1 trials design. | Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 20060301 |
| A randomized, double-blind trial assessing the efficacy and safety of sublingual metopimazine and ondansetron in the prophylaxis of chemotherapy-induced delayed emesis. | Anti-cancer drugs 20060201 |
| Comparison of the efficacy and safety of combinations of metopimazine or ondansetron with methylprednisolone in the prevention of delayed emesis in patients receiving chemotherapy. | Current medical research and opinion 20051101 |
| Options for the prevention and management of acute chemotherapy-induced nausea and vomiting in children. | Paediatric drugs 20030101 |
| Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. | Health and quality of life outcomes 20030101 |
| Ondansetron plus metopimazine compared with ondansetron plus metopimazine plus prednisolone as antiemetic prophylaxis in patients receiving multiple cycles of moderately emetogenic chemotherapy. | Journal of clinical oncology : official journal of the American Society of Clinical Oncology 20010401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.